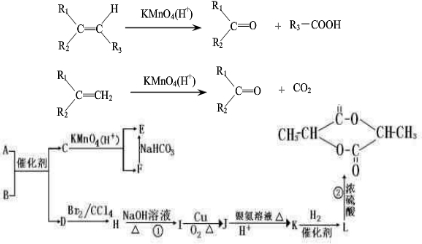
��Ŀ����
��һ����6�֣� ��2��5g̼���ơ�̼�����ƺ��������ƹ���������ȫ�ܽ���ˮ���Ƴ�ϡ��Һ��Ȼ�������Һ����μ���1mol/L�����ᣬ�������������������CO2���������״������ϵ����ͼ��ʾ��
��1��д��OA����������Ӧ�����ӷ���ʽ
��2��������35mL����ʱ������������̼�����Ϊ mL����״����
��3��ԭ�������Na2CO3����������Ϊ ��
��������������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ���α�����ܵĺϳɰ������й��������ǵ¹���ѧ�ҹ�����1905�귢���ģ���ϳ�ԭ��Ϊ��N2(g) + 3H2(g)![]() 2NH3(g)��
2NH3(g)��
��H���D92.4 kJ/mol������˻����1918���ŵ������ѧ�����Իش��������⣺
�� ���з������ʺ�ʵ������ȡ�������� ������ţ���
A������ʯ���е���Ũ��ˮ B������Ũ��ˮ
C��ֱ���������͵����ϳ� D�����Ȼ����Һ�е���Ũ����������Һ
�� �ϳɰ���ҵ�в�ȡ�����д�ʩ������������ԭ�����͵��� ������ţ���
A�����ýϸ�ѹǿ��20 M Pa��50 M Pa��
B������500��ĸ���
C��������ý������
D�������ɵİ�Һ������ʱ����ϵ�з��������δ��Ӧ��N2��H2ѭ�����ϳ�����
��3�� �����ֻ���ϢϵͳDIS������ͼ����ʾ�����ɴ����������ݲɼ����ͼ������ɣ����Բⶨ������ˮ��Ũ�ȡ�����ʽ�ζ���ȷ��ȡ0.5000 mol/L������Һ25.00 mL���ձ��У��Ը��ְ�ˮ���еζ����������Ļ����ʾ����Һ���������氱ˮ����仯����������ͼ����ʾ��


ͼ�� ͼ��
�� �õζ���ʢ��ˮǰ���ζ���Ҫ�� ��ϴ2��3�飬
�� �Լ�����ְ�ˮ��Ũ�ȣ� ��
�� ��������£��ᵼ��ʵ����c(NH3��H2O)ƫ�͵��� ��
A���ζ�����ʱ���Ӷ���
B����ȡ25.00 mL������Һʱ��δ����ʢ��Һ��ϴ�ζ���
C���ζ�ʱ����������ˮ�����ձ���
��4�� 1998��ϣ������ʿ��´�ѧ��Marnellos��Stoukides���ø����ӵ����Ե�SCY�մɣ��ܴ���H+����ʵ���˸��³�ѹ�¸�ת���ʵĵ绯ѧ�ϳɰ�����ʵ��װ������ͼ��

�����ĵ缫��ӦʽΪ�� ��
��һ������6�֣�
��1��H++OH-=H2O��1�� �� CO32-+H+=HCO3-��1�� ��
��2�� 224 ��2�� �� ��3��42��4%��2�� ��
����������12�֣�
�� C D��2�֣� �� AD��2�֣� ��3�����������ⰱˮ �� 2�֣�
�� 0.6250 mol/L��2�֣��� A C ��2�֣� (4) N2+ 6e����2N3����2�֣�
����:��

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д��ش������й������֪ʶ��
��һ����ĸϸ���Ĺ̶�����
��1���Ʊ��̶�����ĸϸ������ �������� ����ԭ������������������������������
��2���Ʊ��̶�����ĸϸ���Ĺ���Ϊ
��ʹ�ɽ�ĸ�� ���� ��ϲ����裬ʹ��ĸ�����
�ڽ���ˮCaCl2�ܽ�������ˮ�У���� CaCl2��Һ��
���þƾ��Ƽ������ƺ���������Һ��
�ܺ���������Һ��ȴ�������ټ����ѻ�Ľ�ĸϸ������ֽ��貢��Ͼ��ȣ�
����ע�����������������������������������������������Ļ���ﻺ�������Ȼ�����Һ�С�
��30min����Դ�CaCl2��Һ��ȡ�������顣����ж������������ijɹ����������������������
������������������������������������������������������������������������
������Ϊ�˼������ˮ���Ƿ��д˾������������䷽���±���
| ������ | ���� | ����[ | K2HPO4 | ����ˮ |
| 10g | 5g | 5g | 2g | 1000mL |
��1������©����������____________________���������������������ʣ���������ӦΪ____________________��
��2����������������̼Դ��_________________���书����___________________������������ʱ������ע�����Ӫ�����ʵ�__________��__________��
��3�����ۺ������������ڸ��ֳɷֶ��ܻ����װǰ��Ҫ���е���__________________��
��4�����˾�����ij��л����X��������ʱ��X����øY��ϣ�����Y�Ľṹ__________�仯��Y�Ļ��Խ��͡��˹����������л�Ĵ�ʩ�����ı�������Ŵ����ԡ�__________�ȡ�
�ش������й������֪ʶ��
��һ����ĸϸ���Ĺ̶�����
��1���Ʊ��̶�����ĸϸ������ �������� ����ԭ������������������������������
��2���Ʊ��̶�����ĸϸ���Ĺ���Ϊ
��ʹ�ɽ�ĸ�� ���� ��ϲ����裬ʹ��ĸ�����
�ڽ���ˮCaCl2�ܽ�������ˮ�У���� CaCl2��Һ��
���þƾ��Ƽ������ƺ���������Һ��
�ܺ���������Һ��ȴ�������ټ����ѻ�Ľ�ĸϸ������ֽ��貢��Ͼ��ȣ�
����ע�����������������������������������������������Ļ���ﻺ�������Ȼ�����Һ�С�
��30min����Դ�CaCl2��Һ��ȡ�������顣����ж������������ijɹ����������������������
������������������������������������������������������������������������
������Ϊ�˼������ˮ���Ƿ��д˾������������䷽���±���
|
������ |
���� |
����[ |
K2HPO4 |
����ˮ |
|
10g |
5g |
5g |
2g |
1000mL |
��1������©����������____________________���������������������ʣ���������ӦΪ____________________��
��2����������������̼Դ��_________________���书����___________________������������ʱ������ע�����Ӫ�����ʵ�__________��__________��
��3�����ۺ������������ڸ��ֳɷֶ��ܻ����װǰ��Ҫ���е���__________________��
��4�����˾�����ij��л����X��������ʱ��X����øY��ϣ�����Y�Ľṹ__________�仯��Y�Ļ��Խ��͡��˹����������л�Ĵ�ʩ�����ı�������Ŵ����ԡ�__________�ȡ�


 +2H2O
+2H2O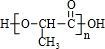 +��n-1��H2O
+��n-1��H2O