��Ŀ����
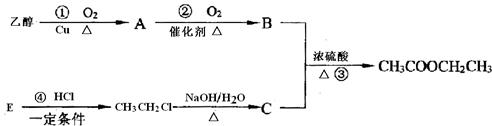
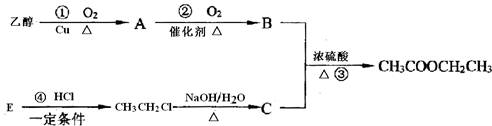
EΪ������������д�����з�Ӧ�Ļ�ѧ����ʽ���л����ýṹ��ʽ��ʾ������ָ����Ӧ�ķ�Ӧ���ͣ�
��A��B
��B+D��E
��A��C

��A��B
CH3CHO+H2
CH3CH2OH
| ���� |
| �� |
CH3CHO+H2
CH3CH2OH
��Ӧ���ͣ�| ���� |
| �� |
�ӳɷ�Ӧ
�ӳɷ�Ӧ
����B+D��E
CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O
| Ũ���� |
| �� |
CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O
��Ӧ���ͣ�| Ũ���� |
| �� |
������Ӧ
������Ӧ
����A��C
CH3CHO+2Ag��NH3��2OH
CH3COONH4+3NH3��+2Ag��+H2O
| �� |
CH3CHO+2Ag��NH3��2OH
CH3COONH4+3NH3��+2Ag��+H2O
��Ӧ���ͣ�| �� |
������Ӧ
������Ӧ
��
������EΪ������������B��DӦ�ֱ�ΪCH3CH2OH��CH3COOH����ת����ϵ��֪B��A�����������ӳɷ�Ӧ���ɣ���BӦΪCH3CH2OH����AΪCH3CHO��DΪCH3COOH��CӦΪCH3COONH4����϶�Ӧ���ʵ������Լ���ĿҪ��ɽ����⣮
����⣺EΪ������������B��DӦ�ֱ�ΪCH3CH2OH��CH3COOH����ת����ϵ��֪B��A�����������ӳɷ�Ӧ���ɣ���BӦΪCH3CH2OH����AΪCH3CHO��DΪCH3COOH��
CӦΪCH3COONH4����
��AΪCH3CHO�������ڴ����������·����ӳɷ�Ӧ������CH3CH2OH����Ӧ�ķ���ʽΪCH3CHO+H2
CH3CH2OH���ʴ�Ϊ��CH3CHO+H2
CH3CH2OH���ӳɷ�Ӧ��
��B��DӦ�ֱ�ΪCH3CH2OH��CH3COOH��������Ũ���������¼��ȷ���������Ӧ����������������Ӧ�ķ���ʽΪCH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��������Ӧ��
�ʴ�Ϊ��CH3CH2OH+CH3COOH
CH3COOCH2CH3+H2O��
��CH3CHO����������Һ����������ԭ��Ӧ����Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH
CH3COONH4+3NH3��+2Ag��+H2O��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
CH3COONH4+3NH3��+2Ag��+H2O��������Ӧ��
CӦΪCH3COONH4����
��AΪCH3CHO�������ڴ����������·����ӳɷ�Ӧ������CH3CH2OH����Ӧ�ķ���ʽΪCH3CHO+H2
| ���� |
| �� |
| ���� |
| �� |
��B��DӦ�ֱ�ΪCH3CH2OH��CH3COOH��������Ũ���������¼��ȷ���������Ӧ����������������Ӧ�ķ���ʽΪCH3CH2OH+CH3COOH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3CH2OH+CH3COOH
| Ũ���� |
| �� |
��CH3CHO����������Һ����������ԭ��Ӧ����Ӧ�ķ���ʽΪCH3CHO+2Ag��NH3��2OH
| �� |
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
| �� |
���������⿼���л�����ƶϣ��������л���֪ʶ���ۺϿ����Ӧ�ã�Ϊ�߿��������ͣ���Ŀ�ѶȲ���ע����շ�Ӧ���ص��Լ�������ʵ����ʣ�ѧϰ��ע�������ط�Ӧ�Ļ�ѧ����ʽ����д��

��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ