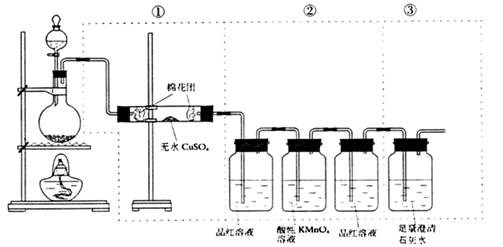
��Ŀ����
��������3С�⣩����10�֣�
I.��2�֣���������ͼʾ��A����ʾ����--------------�ȷ�Ӧ��B����ʾ����--------------�ȷ�Ӧ��
A B
II����2�֣���������������Ӧ�����Ȼ�������ķ�Ӧ�У�������1mol H - H��Ҫ����436KJ������������1mol Cl- Cl��Ҫ����243KJ������������1molH - Cl��Ҫ����432KJ�����������ʾ1mol �����������г��ȼ�յ��Ȼ�ѧ����ʽ�� ��
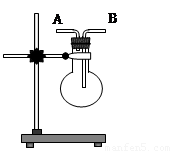
III.��5�֣���ͼ��ʾ�����Թܷ���ʢ��25��ʱ����ʯ��ˮ���ձ��У��Թ��п�ʼ���뼸С��þƬ�����õιܵ���5ml�������Թ��У��Իش��������⣺

(1)ʵ���й۲쵽���������Թ��� ���ձ����������塣
(2)д���Թ��з�Ӧ�����ӷ�Ӧ����ʽ ��
(3)�ɴ���֪��MgCl2��Һ��H2�������� ������ڡ���С �ڡ����ڡ���þƬ�������������
����10�֣�
I. �ţ�1�֣�������1�֣�
II�� H2��g�� + Cl2��g��= 2HCl��g������H = - 185KJ/ mol����2�֣�
III. (1)þƬ���д������ݲ�����þƬ���ܽ⣻��2�֣�
(2)Mg+2H��=Mg2��+H2��2�֣�
(3)С�ڣ�2�֣�
����: