��Ŀ����
ǿ����ǿ���ϡ��Һ�����кͷ�Ӧ����ЧӦΪ��H+(aq)+OH-(aq)�TH2O��l����H=-57.3kJ/mol���ֱ���1L 0.5mol/L��NaOH��Һ�м��룺��ϡ�����Ũ�����ϡ���ᣬǡ����ȫ��Ӧʱ��ЧӦ�ֱ�Ϊ��H1����H2����H3�����ǵĹ�ϵ��ȷ����
A����H1����H2����H3 B����H1>��H3>��H2
C����H1<��H2<��H3 D����H1����H3����H2
ʵ��������һδ֪Ũ�ȵ�ϡ���ᣬijѧ��Ϊ�ⶨ�����Ũ����ʵ�����н�������ʵ�飮�������������գ�
������100mL 0.10mol/L NaOH����Һ��
��ȡ20.00mL����ϡ���������ƿ�У����μ�2��3�η�̪��Һ��ָʾ�����õı�NaOH��Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
ʵ���� | NaOH��Һ��Ũ�ȣ�mol/L�� | NaOH��Һ�������mL�� | ����������Һ�������mL�� |
1 | 0.10 | 20.00 | |
2 | 0.10 | 22.46 | 20.00 |
3 | 0.10 | 22.54 | 20.00 |
��1����1��ʵ��ζ�ǰҺ����0�̶ȣ��ζ�����ͼ��ʾ�����һ��ʵ��ζ�����ȥNaOH��Һ�������¼Ϊ_________mL���ζ��ﵽ�յ��������_____________________��
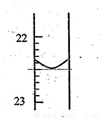
��2�������������ݣ��ɼ�����������Ũ��Ϊ___________��
��3��������ʵ���У����в�������ɲⶨ���ƫ�ߵ���___________
A���ζ��յ�ʱ���Ӷ���
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���ϡ������Һ��ϴ
C����ƿˮϴ��δ����
D������NaOH����Һ������ʱ����
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ


 Fe��s����CO2��g����ƽ�ⳣ��K��0.25������2 L�ܱ������м���0.02mol FeO��s������ͨ��xmolCO��t��ʱ��Ӧ�ﵽƽ�⡣��ʱFeO��s��ת����Ϊ50%����x��ֵΪ�� ��
Fe��s����CO2��g����ƽ�ⳣ��K��0.25������2 L�ܱ������м���0.02mol FeO��s������ͨ��xmolCO��t��ʱ��Ӧ�ﵽƽ�⡣��ʱFeO��s��ת����Ϊ50%����x��ֵΪ�� ��
 B(g) + C(g)����H = -48.25 kJ.mol-1����Ӧ������B��A��Ũ�ȱ���ʱ��t��ͼ��ʾ��ϵ������õ�15minʱc(B)=1.6 mol.L-1�������н�����ȷ����
B(g) + C(g)����H = -48.25 kJ.mol-1����Ӧ������B��A��Ũ�ȱ���ʱ��t��ͼ��ʾ��ϵ������õ�15minʱc(B)=1.6 mol.L-1�������н�����ȷ����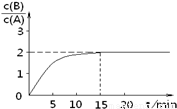
 ɢ��Һ���ɢ���У����û������е�������
ɢ��Һ���ɢ���У����û������е�������