��Ŀ����
���Ṥҵ�з�����Ϊ����������ɷ�ΪSiO2��Fe2O3��Al2O3��MgO��ij̽����ѧϰС���ͬѧ������·����������������н���Ԫ�ص���ȡʵ�顣
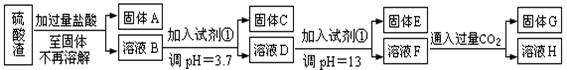
��֪��ҺpH=3.7ʱ��Fe3+�Ѿ�������ȫ��һˮ�ϰ����볣��Kb=1.8��10��5���䱥����Һ��c(OH��)ԼΪ1��10-3mol��L-1����ش�
��1��д��A������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��2����������������ʹ���Լ��٣��Ʋ��Լ���Ӧ���� ����������ĸ��ţ�
��3����ҺD������E��������Ҫ������ҺpH=13�����pH��С�����ܵ��µĺ���� ������дһ�㣩
��4��H�����ʵĻ�ѧʽ�� ��
��5��������ҺF��c(Mg2+)�� �� 25��ʱ��������þ��Ksp=5.6��10-12��

��֪��ҺpH=3.7ʱ��Fe3+�Ѿ�������ȫ��һˮ�ϰ����볣��Kb=1.8��10��5���䱥����Һ��c(OH��)ԼΪ1��10-3mol��L-1����ش�
��1��д��A������������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��2����������������ʹ���Լ��٣��Ʋ��Լ���Ӧ���� ����������ĸ��ţ�
| A���������� | B�������� | C����ˮ | D��ˮ |
��4��H�����ʵĻ�ѧʽ�� ��
��5��������ҺF��c(Mg2+)�� �� 25��ʱ��������þ��Ksp=5.6��10-12��
��1��SiO2+2NaOH=Na2SiO3+H2O��3�֣�
��2��A��2�֣�
��3��þ���ӳ�������ȫ�����������ܽⲻ��ȫ�ȣ�2�֣�
��4��NaHCO3��2�֣�
��5��5.6�� 10�C10 mol/L��3�֣�
��2��A��2�֣�
��3��þ���ӳ�������ȫ�����������ܽⲻ��ȫ�ȣ�2�֣�
��4��NaHCO3��2�֣�
��5��5.6�� 10�C10 mol/L��3�֣�
��

��ϰ��ϵ�д�
�����Ŀ