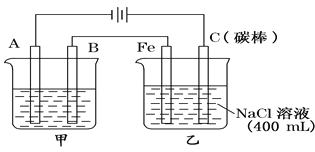
��Ŀ����
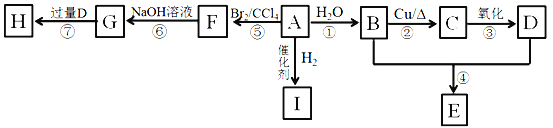
����Ŀ��A��I�dz����л��A������E�ķ���ʽΪC4H8O2��HΪ����ζ����״���ʡ�
��֪��CH3CH2Br��NaOH![]()
��1��0.2mol A��ȫȼ������17.6 g CO2��7.2g H2O����A�Ľṹ��ʽΪ____________��
��2��D�����к��й����ŵ�����Ϊ_____________��
��3���ٵķ�Ӧ����Ϊ____________
��4��G���ܾ��е�����Ϊ__________��
a.���Ʒ�Ӧ b.��NaOH��Һ��Ӧ c.������ˮ
��5����д���ں͢ߵĻ�ѧ����ʽ��
��Ӧ��_________________��
��Ӧ��_________________��
��6��J���л���B��ͬϵ��ұ�B��3��̼ԭ�ӣ�J���ܵĽṹ��___�֣�д�����к�3�������ܵĽṹ��ʽ________________________��
���𰸡� CH2��CH2 �Ȼ� �ӳ� ac 2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O CH2OHCH2OH+2CH3COOH
2CH3CHO+2H2O CH2OHCH2OH+2CH3COOH![]() CH3COOCH2CH2OOCCH3+2H2O 8 (CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3
CH3COOCH2CH2OOCCH3+2H2O 8 (CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3
������������17.6g CO2�����ʵ���Ϊ��n(CO2)=![]() =0.4mol��ˮ�����ʵ���Ϊ
=0.4mol��ˮ�����ʵ���Ϊ![]() =0.4mol��A��һ����������Ԫ���غ��֪0.2mol A��n(C)=0.4mol��n(H)=0.4mol��2=0.8mol����1molA��n(C)=2mol��n(H)=4mol��AΪCH2=CH2��A��ˮ�ӳ�����B��BΪCH3CH2OH����CΪ��ȩ��DΪ���ᣬ�Ҵ������ᷢ��������Ӧ����E��EΪ������������ϩ�������ӳ�����I��IΪ���飻��ϩ����ӳ�����F��FΪ1��2-�������飬 1��2-�������鷢��ˮ������G��GΪ�Ҷ������Ҷ��������ᷴӦ���������Ҷ������ݴ˴��⡣
=0.4mol��A��һ����������Ԫ���غ��֪0.2mol A��n(C)=0.4mol��n(H)=0.4mol��2=0.8mol����1molA��n(C)=2mol��n(H)=4mol��AΪCH2=CH2��A��ˮ�ӳ�����B��BΪCH3CH2OH����CΪ��ȩ��DΪ���ᣬ�Ҵ������ᷢ��������Ӧ����E��EΪ������������ϩ�������ӳ�����I��IΪ���飻��ϩ����ӳ�����F��FΪ1��2-�������飬 1��2-�������鷢��ˮ������G��GΪ�Ҷ������Ҷ��������ᷴӦ���������Ҷ������ݴ˴��⡣
(1)�������Ϸ�����֪AΪ��ϩ��A�Ľṹ��ʽΪCH2=CH2���ʴ�Ϊ��CH2=CH2��
(2)DΪ���Ậ���Ȼ����ʴ�Ϊ���Ȼ���
(3)��Ӧ��ΪCH2=CH2��ˮ��һ�������·����ӳɷ�Ӧ����CH3CH2OH����Ӧ����ʽΪCH2=CH2+H2O![]() CH3CH2OH���ʴ�Ϊ���ӳɷ�Ӧ��
CH3CH2OH���ʴ�Ϊ���ӳɷ�Ӧ��
(4)GΪCH2OHCH2OH��a��������Ʒ�ӦCH2OHCH2OH+2Na��CH2ONaCH2ONa+H2������a��ȷ��b����������NaOH��Һ��Ӧ����b����c���Ҷ���������ˮ����c��ȷ���ʴ�Ϊ��ac��
(5)��Ӧ�ڣ��Ҵ���Cu��Ag�����������·���������Ӧ����ӦΪ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O����Ӧ����CH2OHCH2OH+2CH3COOH
2CH3CHO+2H2O����Ӧ����CH2OHCH2OH+2CH3COOH![]() CH3COOCH2CH2OOCCH3+2H2O���ʴ�Ϊ��2CH3CH2OH+O2
CH3COOCH2CH2OOCCH3+2H2O���ʴ�Ϊ��2CH3CH2OH+O2![]() 2CH3CHO+2H2O�� CH2OHCH2OH+2CH3COOH
2CH3CHO+2H2O�� CH2OHCH2OH+2CH3COOH![]() CH3COOCH2CH2OOCCH3+2H2O��
CH3COOCH2CH2OOCCH3+2H2O��
(6)BΪCH3CH2OH��J���л���B��ͬϵ��ұ�B��3��̼ԭ�ӣ���JΪ�촼������C5H12��3�ֽṹ��CH3CH2CH2CH2CH3��![]() ��
�� ������CH3CH2CH2CH2CH3����3����ԭ�ӣ�����3�ִ���
������CH3CH2CH2CH2CH3����3����ԭ�ӣ�����3�ִ�������4����ԭ�ӣ�����4�ִ���
 ����1����ԭ�ӣ�����1�ִ������J���ܵĽṹ����8�֣����к�3�����Ľṹ��ʽΪ(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3���ʴ�Ϊ��8��(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3��
����1����ԭ�ӣ�����1�ִ������J���ܵĽṹ����8�֣����к�3�����Ľṹ��ʽΪ(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3���ʴ�Ϊ��8��(CH3)3CCH2OH��(CH3)2COHCH2CH3��(CH3)2CHCHOHCH3��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����������������������Ű��������ӱ��ȵ�������������β����ȼú����ɿ�����Ⱦ��ԭ��֮һ������о���������ķ�Ӧ�������������ͷ��λ�����Ⱦ����Ҫ���塣
(1) ����2NO(g) + 2H2(g) = N2(g) + 2H2O(g) ��H = 665 kJ/mol �ķ�Ӧ��������ɣ�
�� 2NO(g) = N2O2(g) (��)
�� N2O2(g) + H2(g) = N2O(g) + H2O(g) (��)
�� ______________________________ (��)������ɵڢ۲��Ļ�ѧ����ʽ����˾������ܷ�Ӧ���ʵ��ǵ�_____���ķ�Ӧ��(�����)
(2) ��֪��H2(g) + CO2(g) = H2O(g) + CO(g) ��H = + 41 kJ/mol����β���ľ���ԭ����Ҫ���ô�����NO��CO��Ӧת��Ϊ���ֶԴ�������Ⱦ�����壬��д���÷�Ӧ���Ȼ�ѧ����ʽ��______________��
�÷�Ӧ��һ�������´ﵽƽ���Ϊ���ܼӿ췴Ӧ���ʲ��÷�Ӧ���������ƶ����ɲ�ȡ�Ĵ�ʩ�У���__________��
A���ʵ������¶� B���ʵ������¶� C��ѹ���������ѹǿ D��ʹ��������
�÷�Ӧ��ȡ������ʩ���´ﵽƽ���Kֵ��______(����������������С������������)
(3) �����¶����þ�������Ļ�ѧ��Ӧ�ӿ췴Ӧ���ʣ������о�����2NO(g) + O2(g) = 2NO2(g) ��H < 0 ����һЩ������������ij��ѧС��ͨ��ʵ���ò�ͬ�¶��¸÷�Ӧ���ʳ���k (������Ӧ���ʵ�һ������)����ֵ���±���
T(K) | k | T(K) | k | T(K) | k |
143 | 1.48 �� 105 | 273 | 1.04 �� 104 | 514 | 3.00 �� 103 |
195 | 2.58 �� 104 | 333 | 5.50 �� 103 | 613 | 2.80 �� 103 |
254 | 1.30 �� 104 | 414 | 4.00 �� 103 | 663 | 2.50 �� 103 |
��ʵ�����ݲv(��)~c(O2)�Ĺ�ϵ��ͼ��ʾ����x�����ߵ�ijһ�¶�ʱ����Ӧ���´ﵽƽ�⣬���Ϊ��Ӧ�ĵ�Ϊ_____��(����ĸ)��������ԭ��__________����__________��