��Ŀ����
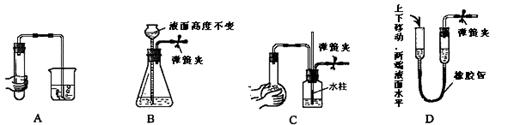
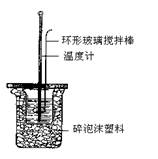
������ʡ������2008������꼶��һѧ�����п��ԣ���ѧ��17����1���к��ȵIJⶨʵ�飨��ͼ����
����ȡ��Ӧ��ʱ��ȡ50mL0.50mol��L-1�����ᣬ��Ӧ������Լ��� ������ţ���
A��50mL0.50mol��L-1NaOH��Һ
B��50mL0.55mol��L-1NaOH��Һ
C��1.0gNaOH����
����ʵ������У���ͬѧ��Ҫ�ⶨ����¼��ʵ�������� ������ţ���
A�������Ũ�� B��������¶�
C������������Һ��Ũ�� D������������Һ���¶�
E��ˮ�ı����� F����Ӧ������Һ����ֹ�¶�
������50mL0.5mol��L-1������Һ������������ⶨ�к��ȣ��������� �����ƫ��ƫС�����䡱��
��2������ͭ���壨CuSO4��xH2O���ᾧˮ�����IJⶨʵ�顣
�ٳ���һ����������Ǧ��������ѽᾧˮʱ�õ���������Ҫ�У������������Ǽܡ�����������ǯ�������Ǻ� ��
��ʵ����Ҫ���������ڸ���������ȴ������ҪĿ����
��
��������������Ϊm������������ͭ���������Ϊm1�����Ⱥ���������ˮ����ͭ������Ϊm2������CuSO4��xH2O��x= ��д����ʽ����
�����ʵ���м����¶ȹ��ߣ�������ɫ��ڣ���ⶨ����� ������䡱��ƫ�ߡ���ƫ�͡�����

����ȡ��Ӧ��ʱ��ȡ50mL0.50mol��L-1�����ᣬ��Ӧ������Լ��� ������ţ���
A��50mL0.50mol��L-1NaOH��Һ
B��50mL0.55mol��L-1NaOH��Һ
C��1.0gNaOH����
����ʵ������У���ͬѧ��Ҫ�ⶨ����¼��ʵ�������� ������ţ���
A�������Ũ�� B��������¶�
C������������Һ��Ũ�� D������������Һ���¶�
E��ˮ�ı����� F����Ӧ������Һ����ֹ�¶�
������50mL0.5mol��L-1������Һ������������ⶨ�к��ȣ��������� �����ƫ��ƫС�����䡱��
��2������ͭ���壨CuSO4��xH2O���ᾧˮ�����IJⶨʵ�顣
�ٳ���һ����������Ǧ��������ѽᾧˮʱ�õ���������Ҫ�У������������Ǽܡ�����������ǯ�������Ǻ� ��
��ʵ����Ҫ���������ڸ���������ȴ������ҪĿ����
��
��������������Ϊm������������ͭ���������Ϊm1�����Ⱥ���������ˮ����ͭ������Ϊm2������CuSO4��xH2O��x= ��д����ʽ����
�����ʵ���м����¶ȹ��ߣ�������ɫ��ڣ���ⶨ����� ������䡱��ƫ�ߡ���ƫ�͡�����
��1���� B����B��D��F��ƫС����2���پƾ��ƣ��ڷ�ֹ��ˮ����ͭ�ڿ���������ˮ����ʹ����������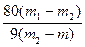 ����ƫ�ߡ�
����ƫ�ߡ�
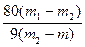 ����ƫ�ߡ�
����ƫ�ߡ���1���ٻ�Ӧ������Լ�ӦΪ���������Զ��ϡNaOH��Һ����ѡB��������к��ȼ��㹫ʽQ����cm(t1��t2)��֪����Ҫ��¼��Һ���¶ȣ���ΪB������¶ȡ�D����������Һ���¶ȡ�F��Ӧ������Һ����ֹ�¶ȣ������ڴ�����������ʣ�����ʱ�����ȣ�������õ����ݻ�ƫС����2���ټ����ѽᾧˮʱ�õ���������Ҫ���˲����������Ǽܡ�����������ǯ�������ǣ�����ƾ��ƣ��ڽ��������ڸ���������ȴ������ҪĿ���Ƿ�ֹ��ˮ����ͭ�ڿ���������ˮ����ʹ�������������ݼ����֪��x=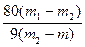 ������ʵ���м����¶ȹ��ߣ�������ɫ��ڣ�������ͭ���·ֽ���������ͭ��ˮ������ƫ�ߣ�Xֵ��ƫ�ߡ�
������ʵ���м����¶ȹ��ߣ�������ɫ��ڣ�������ͭ���·ֽ���������ͭ��ˮ������ƫ�ߣ�Xֵ��ƫ�ߡ�
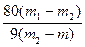 ������ʵ���м����¶ȹ��ߣ�������ɫ��ڣ�������ͭ���·ֽ���������ͭ��ˮ������ƫ�ߣ�Xֵ��ƫ�ߡ�
������ʵ���м����¶ȹ��ߣ�������ɫ��ڣ�������ͭ���·ֽ���������ͭ��ˮ������ƫ�ߣ�Xֵ��ƫ�ߡ�
��ϰ��ϵ�д�
�����Ŀ