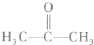
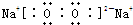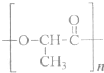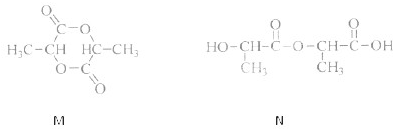��Ŀ����
��16�֣�ij���ӻ�����X��һ���л������Ӻ�һ������������ɣ�Ԫ�ط����õ�H��5.33%��C��21.17%��N��6.17%��S��35.33%��Ge��32.00%���������Ӻ������Ӷ��������������͵Ŀռ�Գ��ԡ�
1��ȷ��X�����ʽ���������ӷֿ�д����
2�����������ӵĿռ乹�ͣ�
һ��������X����HgI2��Ӧ��ͨ������װ�ϳ���������Y��Y����������ͨ��X�������Ӻ�HgS4���������干��S�γɵ�������״�ṹ��
3��д���ϳ�Y�ķ�Ӧ����ʽ��
4��2,3�����ϻ������ںϳ�Y���Dz���ȱ�ٵģ������������ʲô��
5��Y����������ͨ��1��X�е������Ӻͼ�����2��3��4��Hg�����ģ�˵�����ɣ�
���䷨��øþ���Y�����ķ���ϵ����������a��0.9269nm��c��1.4374nm��Z��2��
6�����㾧��Y���ܶȡ�
����Ʒ������DSC��TG�о�����320�渽����һ��ǿ�����ȷ壬�����ͼ��
7����ȷ���������е���Ҫ�ɷ���ʲô��
1��[(CH3)4N]4Ge4S10��3�֣����ʽ��1�֣�
2��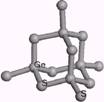 ��2�֣�
��2�֣�
3��[(CH3)4N]4Ge4S10��HgI2��[(CH3)4N]2HgGe4S10��2(CH3)4NI��1�֣�
4����Hg2���γ��ȶ��Ŀ����������������ã�ͬʱҲ��ֹ������HgS���������ɣ���1�֣�
5��ÿ��Ge4S10��3��HgS4������ÿ��HgS4��3��Ge4S10������1.5�֣�
4������ʱΪ��������ṹ��2������ʱΪ�������ͽṹ����1�֣�
�ο�ͼ�Σ�
6��V��a2c��1.2349nm3��1�֣� �ѣ�ZM/NAV��2.58g/cm3��2�֣�
7��GeS2��GeS2��GeS����2.5�֣�

 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д���8�֣����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
| Ԫ�ر�� | Ԫ�����ʻ�ԭ�ӽṹ |
| T | M������2�ԳɶԵ��� |
| X | �����������Ǵ�����������2�� |
| Y | �����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
| Z | Ԫ����������ǣ�7�� |
��2��Ԫ��Y����Ԫ�ؿ��γ�����YH
 ��YH
��YH ���ӵĽṹʽΪ ��
���ӵĽṹʽΪ ����3��Ԫ��Z��T��ȣ��ǽ����Խ�ǿ����_______����Ԫ�����ƣ������б�������֤����һ��ʵ����________��
a.������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b.Z���⻯���T���⻯���ȶ�
c.һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
d.Z������H2����
e.Z��T�����������ϣ���Fe����Z���ɵĻ������м�̬����
��4��д������������ij����Ԫ����ɵķǼ��Է��ӵĽṹʽ
�� ��
��8�֣����в��ֶ�����Ԫ�ص����ʻ�ԭ�ӽṹ���±���
|
Ԫ�ر�� |
Ԫ�����ʻ�ԭ�ӽṹ |
|
T |
M������2�ԳɶԵ��� |
|
X |
�����������Ǵ�����������2�� |
|
Y |
�����µ���Ϊ˫ԭ�ӷ��ӣ����⻯��ˮ��Һ�ʼ��� |
|
Z |
Ԫ����������ǣ�7�� |
��1��Ԫ��T��ԭ������㹲��______�ֲ�ͬ�˶�״̬�ĵ��ӡ�Ԫ��X��һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����_________��
��2��Ԫ��Y����Ԫ�ؿ��γ�����YH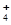 ��YH
��YH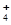 ���ӵĽṹʽΪ
��
���ӵĽṹʽΪ
��
��3��Ԫ��Z��T��ȣ��ǽ����Խ�ǿ����_______����Ԫ�����ƣ������б�������֤����һ��ʵ����________��
a.������Z�ĵ��ʺ�T�ĵ���״̬��ͬ
b.Z���⻯���T���⻯���ȶ�
c.һ��������Z��T�ĵ��ʶ���������������Һ��Ӧ
d.Z������H2����
e.Z��T�����������ϣ���Fe����Z���ɵĻ������м�̬����
��4��д������������ij����Ԫ����ɵķǼ��Է��ӵĽṹʽ
�� ��