��Ŀ����
�л����(C13H18O2)��һ�����ϣ���ϳ�·����ͼ��ʾ������A����Է�������ͨ���������Ϊ56�����ĺ˴Ź���������ʾֻ������壻D���Է���������Ӧ���ڴ�������������1 mol D��2 mol H2��Ӧ���������ң����к�������-CH3
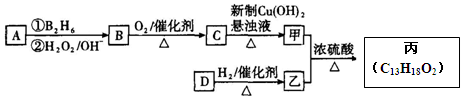
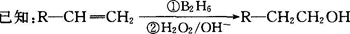
��1��A�Ľṹ��ʽΪ ���ҵķ���ʽΪ ��
��2��C������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ_________________��
��3��D���������ŵ������� ��D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹���� �֣������������칹����
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��5��д�����������������л���Ľṹ��ʽ �����һ�Ϊͬ���칹�壻����FeCl3��Һ����ɫ�����䱽���ϵ�һ�����ֻ�����֡�

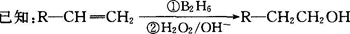
��1��A�Ľṹ��ʽΪ ���ҵķ���ʽΪ ��
��2��C������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ_________________��
��3��D���������ŵ������� ��D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹���� �֣������������칹����
��4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ ��
��5��д�����������������л���Ľṹ��ʽ �����һ�Ϊͬ���칹�壻����FeCl3��Һ����ɫ�����䱽���ϵ�һ�����ֻ�����֡�
��1��(CH3)2C=CH2 C9H12O
��2��(CH3)2CHCHO+2Cu(OH)2+NaOH (CH3)2CHCOONa+Cu2O��+3H2O
(CH3)2CHCOONa+Cu2O��+3H2O
��3��̼̼˫����ȩ�� 4
��4��
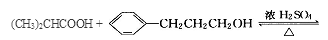
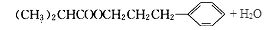
��5��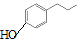
��2��(CH3)2CHCHO+2Cu(OH)2+NaOH
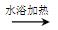 (CH3)2CHCOONa+Cu2O��+3H2O
(CH3)2CHCOONa+Cu2O��+3H2O��3��̼̼˫����ȩ�� 4
��4��
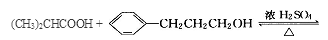
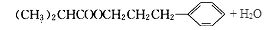
��5��


�����������1����A�����ʼ���Ŀ�ṩ����Ϣ��֪AΪ�����͵�ϩ������ȥ����ʽΪCnH2n��14n=56�����n=4����AΪC4H8����Ϊ���ĺ˴Ź���������ʾֻ������壬˵����������Hԭ�ӣ���AΪCH2=C(CH3)2�� BΪ2-������ (CH3)2CHCH2OH�� B������ΪC��2-����ȩ (CH3)2CHCHO��C�����Ƶ�������ͭ����Һ���ȣ���пɵüף�2-������ (CH3)2CHCOOH����Ϊ���ҷ���������Ӧ�õ���C13H18O2��ˮ���������ҵķ����к��еĸ���Ԫ�ص�ԭ�Ӹ���ΪC��13-4=9��H��18+2-8=12��O��2+1-1=2���ҷ���ʽΪC9H12O����2��2-����ȩ (CH3)2CHCHO������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ(CH3)2CHCHO+2Cu(OH)2+NaOH
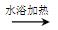 (CH3)2CHCOONa+Cu2O��+3H2O��3��D���Է���������Ӧ��֤��D�к���ȩ����-CHO�����ڴ�������������1 mol D��2 mol H2��Ӧ���������ң������л�����̼̼˫�������D���������ŵ�������̼̼˫����ȩ����D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹����4�֡����Ƿֱ��ǣ�
(CH3)2CHCOONa+Cu2O��+3H2O��3��D���Է���������Ӧ��֤��D�к���ȩ����-CHO�����ڴ�������������1 mol D��2 mol H2��Ӧ���������ң������л�����̼̼˫�������D���������ŵ�������̼̼˫����ȩ����D�ж���ͬ���칹�壬��������������������ͬ��ͬ���칹����4�֡����Ƿֱ��ǣ�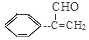 ��
��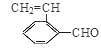 ��
��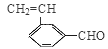 ��
��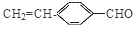 ��
����4�������ҷ�Ӧ�Ļ�ѧ����ʽΪ
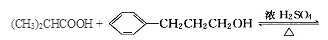
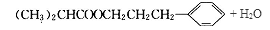
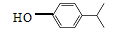
��5��д�����������������һ�Ϊͬ���칹�壻����FeCl3��Һ����ɫ�����䱽���ϵ�һ�����ֻ�����ֵ��л���Ľṹ��ʽ
 ��
�� ��
��
��ϰ��ϵ�д�
 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�
�����Ŀ

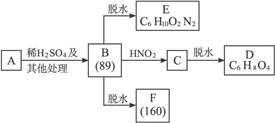
 ���ܷ����ķ�Ӧ������
���ܷ����ķ�Ӧ������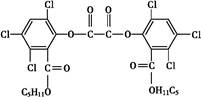
 3�Ǵ����ٴ�����ε�С���ӿ���ҩ��,���ӽṹ��ͼ������˵����ȷ���ǣ� ��
3�Ǵ����ٴ�����ε�С���ӿ���ҩ��,���ӽṹ��ͼ������˵����ȷ���ǣ� ��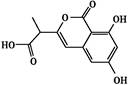
 ��ʾ���ʵ�Ħ�������������и�ʽ����ȷ����
��ʾ���ʵ�Ħ�������������и�ʽ����ȷ���� (��)��
(��)��