��Ŀ����
��21�֣���1����50mL 0.55mol/L NaOH��Һ��50mL 0.25mol/L H2SO4��Һ�����к��Ȳⶨ��ʵ�飬�����Һ�ڷ�Ӧǰ����¶ȱ仯Ϊt1�桫t2�棨t2>t1��,��Ϻ���Һ�ı�����Ϊc = 4.18J����g���棩,��Һ���ܶȶ�����Ϊ1g/mL���к��ȡ�H=__________(�����ʽ�����û���)������H2SO4��Һ������ͬ���ʵ���Ũ�ȵ�CH3COOH��Һ����õġ�H______��(��ƫ����ƫС������ͬ����������ϡ���ỻ��Ũ����������ʵ�飬��õġ�H_______���ƫ����ƫС������ͬ������
��2��ij���ܻ�ѧ����N2H2�ڣ���Ԫ�ص��ӻ�����Ϊ____������ʽΪ____��һ���������ЦҼ� �� ���� �� ����
��3�������ܱ������н��п��淴Ӧ�� CO(g)��NO2(g)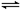 CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�
CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�

�����������������ƽ�������ƶ�(�������������)����Ӧ��������ɫ��������(��������dz�������䡱)
������������䣺��ͨ��CO2���壬ƽ���� ���ƶ�����Ӧ��������ɫ��������ͨ��N2���壬ƽ���� ���ƶ�����Ӧ��������ɫ�������ۼ��������ƽ���������ƶ���
(4)�±��Ǽ��ֳ���ȼ��(1 mol)��ȫȼ��ʱ�ų���������
�ٴ������Ƕȷ�����Ŀǰ���ʺϼ�ͥʹ�õ���������ȼ����________��
��д���ܵ�ú���е�һ����̼ȼ���ȵ��Ȼ�ѧ����ʽ_______________________
�۳��ȼ��1 mol���и���ȼ�ϣ��ŷų�������̼����������________��
�ܿ���ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ��________�ȡ�
��2��ij���ܻ�ѧ����N2H2�ڣ���Ԫ�ص��ӻ�����Ϊ____������ʽΪ____��һ���������ЦҼ� �� ���� �� ����
��3�������ܱ������н��п��淴Ӧ�� CO(g)��NO2(g)
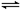 CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�
CO2(g)��NO(g)��(����Ӧ����)���ﵽƽ���ֻ�ı�����һ����������ƽ���Ӱ���ǣ�
�����������������ƽ�������ƶ�(�������������)����Ӧ��������ɫ��������(��������dz�������䡱)
������������䣺��ͨ��CO2���壬ƽ���� ���ƶ�����Ӧ��������ɫ��������ͨ��N2���壬ƽ���� ���ƶ�����Ӧ��������ɫ�������ۼ��������ƽ���������ƶ���
(4)�±��Ǽ��ֳ���ȼ��(1 mol)��ȫȼ��ʱ�ų���������
| ���� | ̿��(C) | һ����̼(CO) | ����(H2) | ����(CH4) | �Ҵ�(C2H5OH) |
| ״̬ | ���� | ���� | ���� | ���� | Һ�� |
| ����(kJ) | 392.8 | 282.6 | 285.8 | 890.3 | 1 367 |
��д���ܵ�ú���е�һ����̼ȼ���ȵ��Ȼ�ѧ����ʽ_______________________
�۳��ȼ��1 mol���и���ȼ�ϣ��ŷų�������̼����������________��
�ܿ���ȼ�ϴ������ޣ�������ȼ�չ����л������Ⱦ��������Դ�������ķ�չս�ԣ��ҹ��������õ���ɫ��Դ��________�ȡ�
��21�֣���1��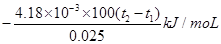 ƫ�� ƫС����2�֣�
ƫ�� ƫС����2�֣�
��2��sp2�ӻ� 3 1 ����1�֣�
��3��������dz ���� ���� �� ���� ����1�֣�
��4��(1)���飨1�֣� (2)CO(g)��1/2O2(g)===CO2(g) ��H����282.6 kJ��mol��1
��2�֣�
(3)�Ҵ���1�֣� (4)���ܡ�̫����(����ܡ�������)��1�֣�
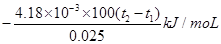 ƫ�� ƫС����2�֣�
ƫ�� ƫС����2�֣���2��sp2�ӻ� 3 1 ����1�֣�
��3��������dz ���� ���� �� ���� ����1�֣�
��4��(1)���飨1�֣� (2)CO(g)��1/2O2(g)===CO2(g) ��H����282.6 kJ��mol��1
��2�֣�
(3)�Ҵ���1�֣� (4)���ܡ�̫����(����ܡ�������)��1�֣�
��1��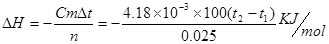 ,CH3COOHΪ���ᣬ����NaOH��Һ��Ӧ�Ĺ����д�������ĵ��룬���������ȣ��ų���������һ���ֱ����գ����Էų����������٣��������ʱ�ǰ����һ���������ò�õ��ʱ�ƫ������ϡ���ỻ��Ũ���ᣬ����Ũ����ϡ�����л���ȣ����Էų��������������ʱ�ǰ��һ���������Բ�õ��ʱ�ƫС��
,CH3COOHΪ���ᣬ����NaOH��Һ��Ӧ�Ĺ����д�������ĵ��룬���������ȣ��ų���������һ���ֱ����գ����Էų����������٣��������ʱ�ǰ����һ���������ò�õ��ʱ�ƫ������ϡ���ỻ��Ũ���ᣬ����Ũ����ϡ�����л���ȣ����Էų��������������ʱ�ǰ��һ���������Բ�õ��ʱ�ƫС��
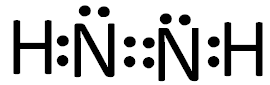
��2��ÿ��Nԭ����Χ��5�����ӣ��ټ�����ԭ�ӵ�һ�����ӣ�ÿ��Nԭ����Χ��6�����ӣ������γ�3�Ե��Ӷԣ����Խ�Ϸ��ӵĽṹ���ж�N��ȡ���ӻ���ʽΪsp2�ӻ�������ʽΪ

���ɵ���ʽ��֪1����������3���Ҽ� ��1���� ����
��3���������ݻ����൱�ڼ�Сѹǿ��ƽ�����������ķ����ƶ������Ǹ÷�Ӧǰ�����û�з����仯�����������ݻ���ƽ�ⲻ�����ƶ����������������ݻ�����������Ũ�ȵļ�С��������ɫ�䵭����ͨ��CO2���壬CO2��Ũ������ƽ���������ƶ�����Ӧ��������ɫ�����ͨ��N2���壬ƽ����ϵ��ѹǿ����ƽ�ⲻ�����ƶ���Ӧ��������ɫ���䡣�۴�����ƽ����Ӱ�����Լ��������ƽ�ⲻ�ƶ���
��4���ٴ������Ƕȷ���������������ȼ�ϼ���ȼ�շų���������࣬���Դ�������飻
��һ����̼ȼ���ȵ��Ȼ�ѧ����ʽCO(g)��1/2O2(g)===CO2(g) ��H����282.6 kJ��mol��1
�۳��ȼ��1 mol���и���ȼ�ϲ����Ķ�����̼����̿��(C)��һ����̼(CO)������(CH4)����1mol,����Ϊ0���Ҵ�Ϊ2mol,���Բ���������̼�������Ҵ���
����ɫ��Դ��̫���ܡ����ܡ����ܡ���ϫ�ܡ������ܡ�
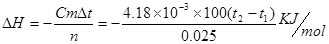 ,CH3COOHΪ���ᣬ����NaOH��Һ��Ӧ�Ĺ����д�������ĵ��룬���������ȣ��ų���������һ���ֱ����գ����Էų����������٣��������ʱ�ǰ����һ���������ò�õ��ʱ�ƫ������ϡ���ỻ��Ũ���ᣬ����Ũ����ϡ�����л���ȣ����Էų��������������ʱ�ǰ��һ���������Բ�õ��ʱ�ƫС��
,CH3COOHΪ���ᣬ����NaOH��Һ��Ӧ�Ĺ����д�������ĵ��룬���������ȣ��ų���������һ���ֱ����գ����Էų����������٣��������ʱ�ǰ����һ���������ò�õ��ʱ�ƫ������ϡ���ỻ��Ũ���ᣬ����Ũ����ϡ�����л���ȣ����Էų��������������ʱ�ǰ��һ���������Բ�õ��ʱ�ƫС��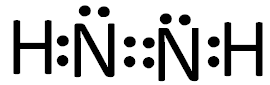
��2��ÿ��Nԭ����Χ��5�����ӣ��ټ�����ԭ�ӵ�һ�����ӣ�ÿ��Nԭ����Χ��6�����ӣ������γ�3�Ե��Ӷԣ����Խ�Ϸ��ӵĽṹ���ж�N��ȡ���ӻ���ʽΪsp2�ӻ�������ʽΪ

���ɵ���ʽ��֪1����������3���Ҽ� ��1���� ����
��3���������ݻ����൱�ڼ�Сѹǿ��ƽ�����������ķ����ƶ������Ǹ÷�Ӧǰ�����û�з����仯�����������ݻ���ƽ�ⲻ�����ƶ����������������ݻ�����������Ũ�ȵļ�С��������ɫ�䵭����ͨ��CO2���壬CO2��Ũ������ƽ���������ƶ�����Ӧ��������ɫ�����ͨ��N2���壬ƽ����ϵ��ѹǿ����ƽ�ⲻ�����ƶ���Ӧ��������ɫ���䡣�۴�����ƽ����Ӱ�����Լ��������ƽ�ⲻ�ƶ���
��4���ٴ������Ƕȷ���������������ȼ�ϼ���ȼ�շų���������࣬���Դ�������飻
��һ����̼ȼ���ȵ��Ȼ�ѧ����ʽCO(g)��1/2O2(g)===CO2(g) ��H����282.6 kJ��mol��1
�۳��ȼ��1 mol���и���ȼ�ϲ����Ķ�����̼����̿��(C)��һ����̼(CO)������(CH4)����1mol,����Ϊ0���Ҵ�Ϊ2mol,���Բ���������̼�������Ҵ���
����ɫ��Դ��̫���ܡ����ܡ����ܡ���ϫ�ܡ������ܡ�

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
�����Ŀ
 ?2NH3��t1ʱ�̴ﵽƽ�⣻t2ʱ���ٴ�A�ڿ��ٳ���һ����NH3�����A��t3ʱ�����´ﵽƽ����t4����0��t4ʱ���ڻ������NH3���������(������)��ʱ��(������)�仯��������ȷ����(��)
?2NH3��t1ʱ�̴ﵽƽ�⣻t2ʱ���ٴ�A�ڿ��ٳ���һ����NH3�����A��t3ʱ�����´ﵽƽ����t4����0��t4ʱ���ڻ������NH3���������(������)��ʱ��(������)�仯��������ȷ����(��)
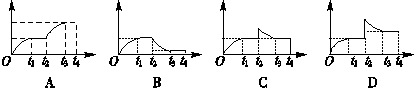
 ,����˵����ȷ���ǣ���
,����˵����ȷ���ǣ��� CO2(g) + H2(g)�� ���ܱ������ﵽƽ�⡣ ���¶�Ϊ749Kʱ��K= 1����CO����ʼŨ����Ϊ2 mol��L-1�� H2O����ʼŨ��Ϊ6mol��L-1ʱ����CO��ת����Ϊ( )
CO2(g) + H2(g)�� ���ܱ������ﵽƽ�⡣ ���¶�Ϊ749Kʱ��K= 1����CO����ʼŨ����Ϊ2 mol��L-1�� H2O����ʼŨ��Ϊ6mol��L-1ʱ����CO��ת����Ϊ( ) 2HI��g������H��0������ͬ�ݻ����ܷ��������ң����м���H2��I2��0��1mol�����м���0��1mol H2��0��2molI2����ͬ�¶��·ֱ�ﵽƽ�⡣����˵������ȷ����
2HI��g������H��0������ͬ�ݻ����ܷ��������ң����м���H2��I2��0��1mol�����м���0��1mol H2��0��2molI2����ͬ�¶��·ֱ�ﵽƽ�⡣����˵������ȷ���� 2C(g) + D(g) ��H>0�������淴Ӧ���ʵ�Ӱ�죬���߽����ʾ����ƽ��ʱ���¶Ȼ�ѹǿ��������ȷ����
2C(g) + D(g) ��H>0�������淴Ӧ���ʵ�Ӱ�죬���߽����ʾ����ƽ��ʱ���¶Ȼ�ѹǿ��������ȷ����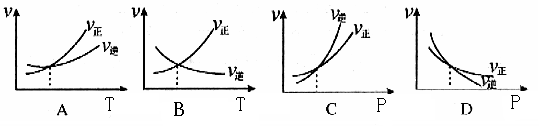
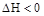 ���ﵽ��ѧƽ�����A�����Ũ��Ϊ
���ﵽ��ѧƽ�����A�����Ũ��Ϊ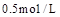 ���������½��ܱ��������ݻ�����һ�����ٴδﵽƽ��ʱ�����A�����Ũ��Ϊ
���������½��ܱ��������ݻ�����һ�����ٴδﵽƽ��ʱ�����A�����Ũ��Ϊ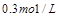 ��������������ȷ���ǣ� ��
��������������ȷ���ǣ� ��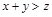
 2NH3(g) ��H =��92.4 kJ��mol-1�����¶ȡ��ݻ���ͬ��2���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�
2NH3(g) ��H =��92.4 kJ��mol-1�����¶ȡ��ݻ���ͬ��2���ܱ������У�����ͬ��ʽͶ�뷴Ӧ����ֺ��¡����ݣ���÷�Ӧ�ﵽƽ��ʱ���й��������£�