��Ŀ����
У�쳧Ư�׳�����22.4L����״����SO2����ѧС��ͬѧ���ݻ�ѧ����ʽZn+2H2SO4��Ũ�� ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4����=1.84g/cm3��110mL��ַ�Ӧ��пȫ���ܽ�����ռ��������������������䣬���Ͼ��ʼ�Ƽ�����������������⣬�����漴���˻أ�
ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4����=1.84g/cm3��110mL��ַ�Ӧ��пȫ���ܽ�����ռ��������������������䣬���Ͼ��ʼ�Ƽ�����������������⣬�����漴���˻أ�
��1����ѧС�����Ƶõ������л��е���Ҫ�������������______�������ʽ�����������ֽ������Ҫԭ����______��
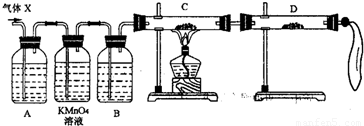
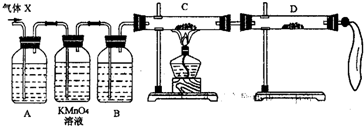
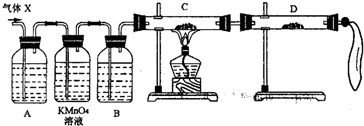
��2��Ϊ֤ʵ��ط�������ѧС���ͬѧ���������ʵ�飬���˻����壨X��ȡ����������̽���������������չ��̣���Ϊȫ���գ���
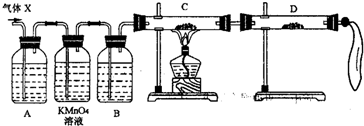
����װ��������ʢ��ҩƷǰ����Ҫ��һ��������______��
��A�м�����Լ������ǣ�______�������ǣ�______��B�м�����Լ������ǣ�______�������ǣ�______��
�ۿ�֤ʵ����X�л��н϶���ij���������ʵ��������______��
��3��������װ���ԼӸĽ����ɴ��ԲⶨX������SO2������������Ľ�ʱ��ѡ�õ���������Ϊ______������ţ���
A�����ܡ� B��ˮ�ۡ� C�����ƿ�� D������ƿ��E����Ͳ�� F���������� G��˫������
�⣺��1�����ŷ�Ӧ�Ľ��У�����Ũ�Ƚ��ͣ�п��ϡ���ᷴӦ��������п��H2����ӦΪ��Zn+H2SO4�TZnSO4+H2����
��˻�ѧС�����Ƶõ������л��е���Ҫ��������������������ʴ�Ϊ��H2��
��2����̽���˻����壨X���ijɷ֣�һ��©������̽�����ʴ�Ϊ�����װ�������ԣ�
�����������Ư��ԭ�����������л�ɫ�ؽ�ϳ���ɫ�IJ��ȶ����������A�м�����Լ�������Ʒ����Һ��������ȷ��SO2���ڣ�Ũ���������ˮ�ԣ���ˮ�����и������ã���ֹ���ӵ�ˮ��װ��D�е�����������ţ�
�ʴ�Ϊ��Ʒ����Һ��ȷ��SO2���ڣ� ŨH2SO4������������
�۸�����Ŀ������װ��ͼ�ͷ�Ӧԭ��֪��װ������ص�װ���Ǽ�����������Ƿ������װ�ã�B��Ũ���ᣬ��ˮ�������ã�C���û�ԭ�����廹ԭ����ͭ��װ�ã�D��ʢ�ŵ�����ˮ����ͭ�������Ƿ���ˮ���ɣ�ֻҪ��ɫ���ɫ����ˮ����ͭ����ɫ����֤�����������ʴ�Ϊ����ɫCuO��ĩ���ɫ����ˮ����ͭ����ɫ��
��3��������Ŀ������װ��ͼ�ͷ�Ӧԭ��֪������C��ֻ������������������ˮ������ˮ���ռ�������Ӧ���ǴӶ̹ܽ����ܳ����ų�ˮ�������������������ʴ�Ϊ��CEG��
��������1��п��ϡ���ᷴӦ����H2��
��2����̽���˻����壨X���ijɷ֣����Բ���©����
�ڸ���SO2��Ư��ԭ�������ͣ�Ũ���������ˮ�������ã�
�۸�����Ŀ������װ��ͼ�ͷ�Ӧԭ�����
��3������ˮ���ⶨ���������
��������������ʵ��̽���⣬��Ҫ����������ļ��飬�ۺ��ԱȽ�ǿ���������Ŀ�ṩ����Ϣ����Ͽα���ѧ����֪ʶ�������
��˻�ѧС�����Ƶõ������л��е���Ҫ��������������������ʴ�Ϊ��H2��
��2����̽���˻����壨X���ijɷ֣�һ��©������̽�����ʴ�Ϊ�����װ�������ԣ�
�����������Ư��ԭ�����������л�ɫ�ؽ�ϳ���ɫ�IJ��ȶ����������A�м�����Լ�������Ʒ����Һ��������ȷ��SO2���ڣ�Ũ���������ˮ�ԣ���ˮ�����и������ã���ֹ���ӵ�ˮ��װ��D�е�����������ţ�
�ʴ�Ϊ��Ʒ����Һ��ȷ��SO2���ڣ� ŨH2SO4������������
�۸�����Ŀ������װ��ͼ�ͷ�Ӧԭ��֪��װ������ص�װ���Ǽ�����������Ƿ������װ�ã�B��Ũ���ᣬ��ˮ�������ã�C���û�ԭ�����廹ԭ����ͭ��װ�ã�D��ʢ�ŵ�����ˮ����ͭ�������Ƿ���ˮ���ɣ�ֻҪ��ɫ���ɫ����ˮ����ͭ����ɫ����֤�����������ʴ�Ϊ����ɫCuO��ĩ���ɫ����ˮ����ͭ����ɫ��
��3��������Ŀ������װ��ͼ�ͷ�Ӧԭ��֪������C��ֻ������������������ˮ������ˮ���ռ�������Ӧ���ǴӶ̹ܽ����ܳ����ų�ˮ�������������������ʴ�Ϊ��CEG��
��������1��п��ϡ���ᷴӦ����H2��
��2����̽���˻����壨X���ijɷ֣����Բ���©����
�ڸ���SO2��Ư��ԭ�������ͣ�Ũ���������ˮ�������ã�
�۸�����Ŀ������װ��ͼ�ͷ�Ӧԭ�����
��3������ˮ���ⶨ���������
��������������ʵ��̽���⣬��Ҫ����������ļ��飬�ۺ��ԱȽ�ǿ���������Ŀ�ṩ����Ϣ����Ͽα���ѧ����֪ʶ�������

��ϰ��ϵ�д�
�����Ŀ
 ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4����=1.84g/cm3��110mL��ַ�Ӧ��пȫ���ܽ�����ռ��������������������䣬���Ͼ��ʼ�Ƽ�����������������⣬�����漴���˻أ�
ZnSO4+SO2��+2H2O�����ȡ65.0gп����98%��ŨH2SO4����=1.84g/cm3��110mL��ַ�Ӧ��пȫ���ܽ�����ռ��������������������䣬���Ͼ��ʼ�Ƽ�����������������⣬�����漴���˻أ�