��Ŀ����
Ϊ�ⶨij�л�������A�Ľṹ����������ʵ�飮
[����ʽ��ȷ��]
��1�����л���A�����������г��ȼ�գ�ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L����״���£�����������и�Ԫ�ص�ԭ�Ӹ�������______��
��2�������Dzⶨ�л����������Է�������Ϊ46��������ʵķ���ʽ��______��
��3������Ԫ�صĻ��ϼۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ______��
[�ṹʽ��ȷ��]
��4�����ⶨ���л���A��������3����ԭ�ӣ���A�Ľṹ��ʽΪ______��
[����ʵ��]
��5��A��ͭ�Ĵ��£��ɱ���������ΪB����Ӧ����ʽΪ��______
B�ڼ��ȵ���������������Һ��Ӧ����Ӧ����ʽΪ��______
��6��A��һ����������ˮ������C��C�ɾۺϳɰ�װ����D����д��Cת��ΪD�Ļ�ѧ��Ӧ����ʽ��______
��7�����������е��˶�Ա����Ť��ʱ����ҽ�漴�������飨�е�Ϊ12.27�棩�����˲�λ���оֲ��䶳����������Cѡ����ʵ��Լ��ͷ����Ʊ������飬Ҫ��ԭ��������Ϊ100%����д���Ʊ���Ӧ����ʽ��______��
��8��A��ͨ����ʳ��һ���������Ƶã�����ʳ�Ƶõ�A��һ���¶����ܱմ��棬��Ϊ����һϵ�еĻ�ѧ�仯����ø����㣮��д�����һ����Ӧ�Ļ�ѧ����ʽ��______��
[����ʽ��ȷ��]
��1�����л���A�����������г��ȼ�գ�ʵ���ã�����5.4gH2O��8.8gCO2����������6.72L����״���£�����������и�Ԫ�ص�ԭ�Ӹ�������______��
��2�������Dzⶨ�л����������Է�������Ϊ46��������ʵķ���ʽ��______��
��3������Ԫ�صĻ��ϼۣ�Ԥ��A�Ŀ��ܽṹ��д���ṹ��ʽ______��
[�ṹʽ��ȷ��]
��4�����ⶨ���л���A��������3����ԭ�ӣ���A�Ľṹ��ʽΪ______��
[����ʵ��]
��5��A��ͭ�Ĵ��£��ɱ���������ΪB����Ӧ����ʽΪ��______
B�ڼ��ȵ���������������Һ��Ӧ����Ӧ����ʽΪ��______
��6��A��һ����������ˮ������C��C�ɾۺϳɰ�װ����D����д��Cת��ΪD�Ļ�ѧ��Ӧ����ʽ��______
��7�����������е��˶�Ա����Ť��ʱ����ҽ�漴�������飨�е�Ϊ12.27�棩�����˲�λ���оֲ��䶳����������Cѡ����ʵ��Լ��ͷ����Ʊ������飬Ҫ��ԭ��������Ϊ100%����д���Ʊ���Ӧ����ʽ��______��
��8��A��ͨ����ʳ��һ���������Ƶã�����ʳ�Ƶõ�A��һ���¶����ܱմ��棬��Ϊ����һϵ�еĻ�ѧ�仯����ø����㣮��д�����һ����Ӧ�Ļ�ѧ����ʽ��______��
��1��5.4gH2O����0.3mol����H��0.6mol��8.8gCO2��0.2mol����C��0.2mol��6.72LO2��0.3mol��0.6molH����0.15molO2��0.2molC����0.2molO2����O2��0.3mol������������0.1mol����n��C����n��H����n��O��=2��6��1��
�ʴ�Ϊ��n��C����n��H����n��O��=2��6��1��
��2����n��C����n��H����n��O��=2��6��1�������л��������ʵ��ʽΪC2H6O���ʴ�Ϊ��C2H6O��
��3��A�Ŀ��ܽṹΪ��CH3CH2OH��CH3-O-CH3���ʴ�Ϊ��CH3CH2OH��CH3-O-CH3��
��4�����л���A��������3����ԭ�ӣ�����A�Ľṹ��ʽΪ��CH3CH2OH���ʴ�Ϊ��CH3CH2OH��
��5�����ݴ��ܱ������������Һ��������ȩ������ʽ�ֱ�Ϊ��2CH3CH2OH+O2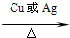 2CH3CHO+2H2O��
2CH3CHO+2H2O��
CH3CHO+2Ag��NH3��2OH
2Ag��+3NH3��+H2O+CH3COONH4���ʴ�Ϊ��2CH3CH2OH+O2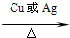 2CH3CHO+2H2O��
2CH3CHO+2H2O��
CH3CHO+2Ag��NH3��2OH
2Ag��+3NH3��+H2O+CH3COONH4��
��6����ϩ�ܷ����Ӿ۷�Ӧ���ɾ���ϩ��nCH2=CH2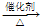
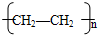 ���ʴ�Ϊ��nCH2=CH2
���ʴ�Ϊ��nCH2=CH2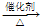
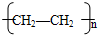 ��
��
��7����ϩ�����Ȼ��ⷢ���Ӿ۷�Ӧ���������飺CH2=CH2+HCl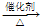 CH3CH2Cl���ʴ�Ϊ��CH2=CH2+HCl
CH3CH2Cl���ʴ�Ϊ��CH2=CH2+HCl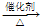 CH3CH2Cl��
CH3CH2Cl��
��8�������ܺ��Ҵ�����������Ӧ��CH3COOH+CH3CH2OH CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
�ʴ�Ϊ��n��C����n��H����n��O��=2��6��1��
��2����n��C����n��H����n��O��=2��6��1�������л��������ʵ��ʽΪC2H6O���ʴ�Ϊ��C2H6O��
��3��A�Ŀ��ܽṹΪ��CH3CH2OH��CH3-O-CH3���ʴ�Ϊ��CH3CH2OH��CH3-O-CH3��
��4�����л���A��������3����ԭ�ӣ�����A�Ľṹ��ʽΪ��CH3CH2OH���ʴ�Ϊ��CH3CH2OH��
��5�����ݴ��ܱ������������Һ��������ȩ������ʽ�ֱ�Ϊ��2CH3CH2OH+O2
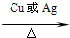 2CH3CHO+2H2O��
2CH3CHO+2H2O��CH3CHO+2Ag��NH3��2OH
| ˮԡ���� |
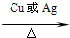 2CH3CHO+2H2O��
2CH3CHO+2H2O��CH3CHO+2Ag��NH3��2OH
| ˮԡ���� |
��6����ϩ�ܷ����Ӿ۷�Ӧ���ɾ���ϩ��nCH2=CH2
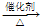
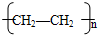 ���ʴ�Ϊ��nCH2=CH2
���ʴ�Ϊ��nCH2=CH2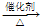
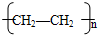 ��
����7����ϩ�����Ȼ��ⷢ���Ӿ۷�Ӧ���������飺CH2=CH2+HCl
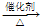 CH3CH2Cl���ʴ�Ϊ��CH2=CH2+HCl
CH3CH2Cl���ʴ�Ϊ��CH2=CH2+HCl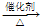 CH3CH2Cl��
CH3CH2Cl����8�������ܺ��Ҵ�����������Ӧ��CH3COOH+CH3CH2OH
 CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOC2H5+H2O���ʴ�Ϊ��CH3COOH+CH3CH2OH CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
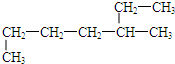 �������ǣ�������
�������ǣ�������