��Ŀ����
���Ȼ���(SCl2)�۵�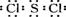
�Իش���������
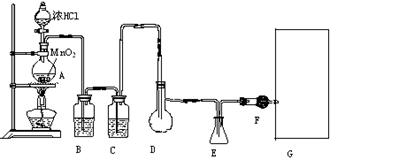
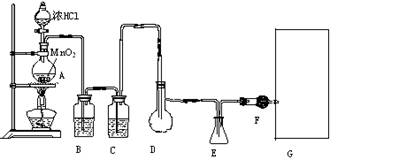
(1)װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ______________________________________________��
(2)ʵ�鿪ʼǰ����D�з�һ��������ۣ�����ʹ���ۻ���Ȼ��ת����ҡ����ƿʹ��������ƿ�ڱ��γ�һ���㣬��������Ŀ����_______________________________________________��
(3)ʵ��ʱ��Dװ���������50��
(4)Fװ���и��������ʢ���ʵ�������_____________________________________________��
(5)���Ȼ������ʽΪ_______________��
(1)MnO2+4HCl(Ũ)![]() MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
(2)����Ӧ�Ӵ���
(3)ˮԡ ����ƿ���˱�ˮ����ȴ
(4)��ֹ������ˮ��������E�в����ղ��������
(5) ![]()

��ϰ��ϵ�д�
�����Ŀ