��Ŀ����
��1��NAΪ�����ӵ�������25��ʱ��1gˮ�к�H+���Ӹ���ԼΪ__________NA��
��2��ͨ�����Ĵ���������ȡ���ᣬ�ڴ�ȫ�����У������ϰ����������������ʵ�����Ϊ__________,���������������������Ϊ_____________��
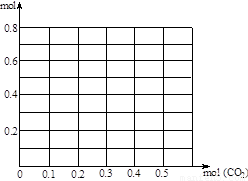
��3������0��2 mol NaOH��0��1 mol Ca(OH)2�Ļ����Һ�г����ȶ���ͨ��CO2����0��5 mol������CO2����Ϊ�����꣬����Һ�����ӵ�����Ϊ�����꣬��������������CO2�������仯������ͼ��(����������ʵĵ�����ε�ˮ��)
��4��ij�о���ѧϰС������ͭм������ͭ������������������ɵĻ��ᷴӦ����ȡCuSO4��5H2O���壬����������Ļ�ԭ����ΪNO����Ӧ�����в�����SO2����Ӧ�����Һ�в���Cu��NO3��2�� ��Ӧ�й�����ȫ�ܽ⣬�������ǡ����ȫ��Ӧ������������������Ϊ480 g������ͭм����������Ϊ0��4�� 480g����������һ���������Ⱥ�ַ�Ӧ����ȴǡ��ֻ�õ�CuSO4��5H2O������ԭ������H2SO4������������д��������̣�
��1��10-10 (2��)
��2��1��2 0��778 (4��)
��3��
(0,0��7) (0��1,0��4) (0��2,0��3) (0��3,0��4) (0��4,0��7) (0��5,0��7) (5��)
��4��(4��) �� m(Cu) = 480��0��4=192g m(CuO)=480g-192g=288g
n(Cu) =192/64=3mol�� n(CuO) = 288/80=3��6mol ��1�֣�
n(Cu) +n(CuO)=3mol +3��6mol = 6��6mol
��Cu�غ�ã� n(H2SO4)=n(CuSO4��5H2O)=6��6mol��1�֣�
m(CuSO4��5H2O)=6��6mol��250g/mol=1650g
��HNO3Ϊxmol ,�ɵ�ʧ��������ͬ�ã� 2��480��0��4/64=3x x=2
n(HNO3) =2mol ��ų�NOΪ2mol������Ϊ2��30=60g��1�֣�
���������غ㣺m(��Һ)=1650+2��30-480=1230g��1�֣�
���ԣ�H2SO4%=6��6��98/12300��100��=52��6%��1�֣�
��������
�����������1��ˮ�������C(H+)=1��0��10-7mol•L‾1, 1gˮ�к�H+Ϊ��1g��1000g/L��1��0��10-7mol•L‾1=10-10mol��
��2�����Ĵ���������ȡ���ᷢ�����·�Ӧ��4NH3+5O2
 4NO+6H2O��4NO+3O2+2H2O=4HNO3,�������������������ʵ�����Ϊ��1:2�����ݻ�ѧ����ʽ���Կ���������4molHNO3�����յ�4molH2O�������������������=4mol��63g/mol��(4mol��63g/mol+4mol��18g/mol)= 0��778��
4NO+6H2O��4NO+3O2+2H2O=4HNO3,�������������������ʵ�����Ϊ��1:2�����ݻ�ѧ����ʽ���Կ���������4molHNO3�����յ�4molH2O�������������������=4mol��63g/mol��(4mol��63g/mol+4mol��18g/mol)= 0��778��
��3��ͨ��CO2ǰ��0��2 mol NaOH��0��1 mol Ca(OH)2�Ļ����Һ��������0��7mol�������������(0,0��7)��ͨ��CO2���ȷ�����Ӧ��CO2+Ca2++2OH‾=CaCO3��+H2O����ȫ��Ӧ����0��1molCO2������0��3mol���ӣ���ת�۵�����(0��1,0��4)������ͨ��CO2��������2����Ӧ��CO2+2OH‾=CO32‾+H2O ,������Ӧ����CO2 0��1mol�����Ӽ���0��1mol���õ�2��ת�۵�(0��2,0��3)����3����Ӧ��CO2+CO32‾+H2O=2HCO3‾������CO2 0��1mol����������0��1mol���õ�3��ת�۵�(0��3,0��4)����4����Ӧ��CO2+CaCO3+H2O=Ca2++2HCO3‾������CO2 0��1mol����������0��3mol���õ�4��ת�۵�(0��4,0��7)����Ϊ����������ʵĵ�����ε�ˮ�⣬��ͨ��CO2������Ũ�Ȳ��䣬���յ�(0��5,0��7)�����Ӹ����ת���ߡ�
��4���������������Ԫ���غ����㣬n(H2SO4)=n(CuSO4��5H2O)=n(Cu)+n(CuO), ��Ŀ�Ѹ����������������������Ϊ480 g��ͭм����������Ϊ0��4���ֱ����Cu��CuO������������������ʵ�����������Һ���������������غ㶨�����㣬��Ӧǰ�������������+������Һ������=��Ӧ��CuSO4��5H2O������+NO���������������H2SO4������������
���㣺���⿼���˻�ѧ�����ͨ���������ͼ��

S8�����γɵĵ�б���б������ͬ�������壬ת����ϵ���£�
S��б�����̣�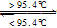 S����б���̣���H=+0.398kJ��mol��1��NAΪ�����ӵ�������������˵���У�������ǣ� ��
S����б���̣���H=+0.398kJ��mol��1��NAΪ�����ӵ�������������˵���У�������ǣ� ��
| A��������б����ȵ�б���ȶ� |
| B����б���б����֮���ת�����������仯 |
| C����б���б�����ڳ����������ȼ�վ�����SO2 ? |
| D��64 g ��б���б����Ļ���ﺬ��ԭ����ĿΪ2NA |
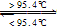 S����б���̣���H=+0.398kJ��mol��1��NAΪ�����ӵ�������������˵���У�������ǣ�
��
S����б���̣���H=+0.398kJ��mol��1��NAΪ�����ӵ�������������˵���У�������ǣ�
��