��Ŀ����
(16��) ͼ����ʵ�����г������Ʊ����������װ��:
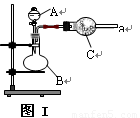
(1) ��ͬѧ��ͼ��װ�á�ͭ��Ũ�����Ʊ����ռ������NO2���壺
�� B�з�Ӧ�Ļ�ѧ����ʽ_____________________________��
�� �ռ�NO2����ķ��� ��
�� ���ռ���NO2����ƿ�ܷ�����ˮ�У���ƿ��������ɫ��dz, �����з�Ӧ��
2NO2
(g)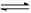 N2O4(g) ��H �еĦ�H 0���>����<����
N2O4(g) ��H �еĦ�H 0���>����<����
��2�� ��ͬѧ��ͼ��װ����ȡNH3��O2�Ļ�����壬��ͼ��װ����֤����ijЩ���ʣ�
��A�м���Ũ��ˮ��B�м���Na2O2���壬C�м����ʯ�ң�D�ڷ��ô�������ʯ�ޣ�������������a�� b��c�� h���Ӹ����� ��
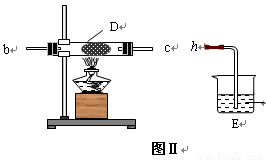
�� ʵ���й۲쵽D���к���ɫ������֣�֤����������____��������ԡ���ԭ�ԡ�����
�� D�з�Ӧ�Ļ�ѧ����ʽΪ________________ _____�� .
�� Ϊ��ֹNO2 ��Ⱦ������Eװ����װ���Լ������� ��
��16�� ��
��1�� ��Cu + 4HNO3���� Cu��NO3��2+ 2NO2��+ 2H2O ��3�֣�
������������ ��2�֣� �� <��2�֣�
��2�� �ٻ�ԭ�� ��2�֣�
�� 4NH3
+ 5O2  4NO +
6H2O ��3�֣���2NO
+ O2 ���� 2NO2 ��2�֣���
4NO +
6H2O ��3�֣���2NO
+ O2 ���� 2NO2 ��2�֣���
�� NaOH��Һ��2�֣�
����������

 ��У����ϵ�д�
��У����ϵ�д�
 ___________________��
___________________�� N2O4(g) ��H �еĦ�H 0���>����<����
N2O4(g) ��H �еĦ�H 0���>����<����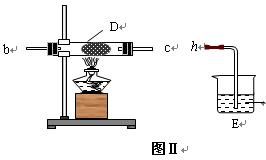
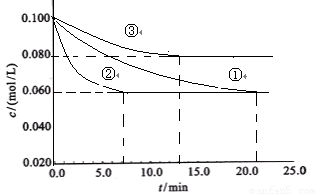
 2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����N2��H2��Ũ�Ⱦ�Ϊc(N2) = 0.100mol/L, c(H2)
= 0.300mol/L�����з�Ӧʱ, N2��Ũ����ʱ��ı仯��ͼ�١��ڡ���������ʾ��
2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����N2��H2��Ũ�Ⱦ�Ϊc(N2) = 0.100mol/L, c(H2)
= 0.300mol/L�����з�Ӧʱ, N2��Ũ����ʱ��ı仯��ͼ�١��ڡ���������ʾ��
 2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����Ũ�Ⱦ�ΪC(N2)=0.100mol/L,
C(H2)=0.300mol/L���з�Ӧʱ, N2��Ũ����ʱ��ı仯����ͼ�١��ڡ���������ʾ��
2NH3 ��H��0��ijʵ������������ͬ�������ܱ������У��ֱ����Ũ�Ⱦ�ΪC(N2)=0.100mol/L,
C(H2)=0.300mol/L���з�Ӧʱ, N2��Ũ����ʱ��ı仯����ͼ�١��ڡ���������ʾ��