��Ŀ����
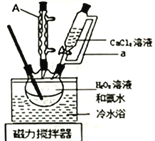
����Ŀ����ѧ��ѧ�м��ֳ������ʵ�ת����ϵ����ͼ��ʾ��
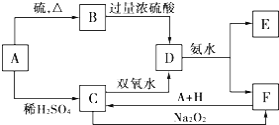
�� D ��Һ�����ˮ�пɵõ��� F Ϊ��ɢ�ʵĺ��ɫ���塣��ش�����������
(1)���ɫ���� F ��ֱ����С�ķ�Χ��_____��
(2)A��B��H �Ļ�ѧʽ��A_____��B_____��H_____��
(3)��д�� C ��������Һ��˫��ˮ��Ӧ�����ӷ���ʽ��_____��
��д������ D �������ӵ�ʵ�鷽��������_____��
���� C ��Һ�м����� C �����ʵ����� Na2O2��ǡ��ʹ C ת��Ϊ F��ͬʱ����һ�ֿ�ʹ������ľ���� ȼ��������д���÷�Ӧ�����ӷ���ʽ��_____��
���𰸡�(1)1��100 nm (2)Fe FeS H2SO4(ϡ) ��ÿ��1�֣�
(3)��2Fe2����H2O2��2H��===2Fe3����2H2O��3�֣�
��ȡ����E���Թ��У��ý�ͷ�ιܵ���NaOH��Һ�������Թܣ��ɹ۲쵽�Թܿڴ�ʪ��ĺ�ɫʯ����ֽ����(������������)��3�֣�
��4Fe2����4Na2O2��6H2O===4Fe(OH)3����O2����8Na����3�֣�
��������

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ