��Ŀ����
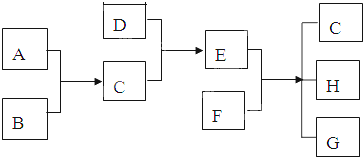
��12�֣���֪A��B��C��DΪ�������ʣ�����B��C��D���³�ѹ��Ϊ���壬�ס��ҡ�������Ϊ�����Ļ�����ҳ�����ΪҺ�壬������ɫ��ӦΪ��ɫ����ͼΪ��������֮���ת����ϵ��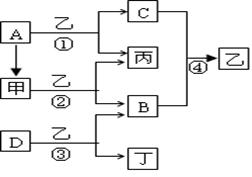
��ش��������⣺
��1��д���������ʵĻ�ѧʽ��
B ��D ��
��2���ĵ���ʽΪ ����Ӧ��������11.2L����״���£�B���ɣ�����ת�Ƶĵ��ӵ����ʵ���Ϊ ��
��3��д����Ӧ�ٵ����ӷ���ʽ�� ��
д����Ӧ�۵Ļ�ѧ����ʽ�� ��
��O2 F2
�ƹ������Ƶ���ʽ�� 1mol
��2Na+2H2O=2Na++2OH��+H2��
2 F2+2 H2O=" 4HF+" O2
����

��ϰ��ϵ�д�
�����Ŀ
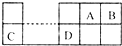 ��֪A��B��C��DΪ����������Ԫ�أ������λ�ù�ϵ��ͼ��C��B���γ����ӻ��� ��C3B2�����з�����ȷ���ǣ�������
��֪A��B��C��DΪ����������Ԫ�أ������λ�ù�ϵ��ͼ��C��B���γ����ӻ��� ��C3B2�����з�����ȷ���ǣ�������| A���縺�ԣ�C��A | B�����������ԣ�C��D | C���⻯���ȶ��ԣ�A��B | D��ԭ�Ӱ뾶��r��C����r��B�� |
 ����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش�
����ͨ������£���A��BΪ���ӣ�C��EΪ�����ӣ�DΪ�����ӣ����Ƕ�����10�����ӣ�B����A�����õ����ʿɵ����C��D��A��B��E��������Ӧ��ɵ�C��һ�ְ�ɫ��������ش� ��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��?
��֪A��B��C��DΪ���壬E��FΪ���壬G���Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��?