��Ŀ����
��Fe��Al��һ������������ɺϽ���1��ȡһ�����ĸúϽ������м�������NaOH��Һ����������n L����״��������Ӧ�����ӷ���ʽ��______���Ͻ���Al�����ʵ�����______��
��2����ȡ��ͬ�����ĸúϽ������м�������ϡ���ᣬ����ȫ���ܽ⣬��������m L ����״��������Ӧ��ת�Ƶ��ӵ����ʵ�����______ mol���Ͻ���Fe��������______��
��3����2��������Һ�м������NaOH��Һ���������ij������ˣ���ϴ�ӡ�������պ�õ�һ�ֹ��壬�ù���������ԭ�Ͻ��������ȣ���ԭ�Ͻ�����������������______��
���𰸡���������1�������������Ʋ���Ӧ����������������Һ��Ӧ����ƫ�����ƺ�������
����n=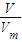 �������������ʵ������������ӷ���ʽ����Al�����ʵ�����
�������������ʵ������������ӷ���ʽ����Al�����ʵ�����
��2������n=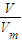 �������������ʵ��������ݵ���ת���غ����ת�Ƶ������ʵ�����
�������������ʵ��������ݵ���ת���غ����ת�Ƶ������ʵ�����
����������ϡ���ᣬFe��Al�����ᶼ��Ӧ����������Al���ɵ���������������������Ʒ�Ӧ���ɵ�����������ݴ˼���Fe�����ᷴӦ������������������ݵ���ת�ƿ�֪n��Fe��=n��H2�����ٸ���m=nM���㣻
��3����2�����õ���Һ���������NaOH��Һ��������ת��Ϊƫ����������������������������պ����ù���Ϊ�������������������ԭ����������ǡ����ȣ�������������Ԫ�ص��������ڻ������Al���������ݴ˽���������Ļ�ѧʽ����ԭ�������Al������������
����⣺��1�������������Ʋ���Ӧ����������������Һ��Ӧ����ƫ�����ƺ���������Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
n L���������ʵ���=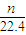 mol���ɷ���ʽ��֪n��Al��=
mol���ɷ���ʽ��֪n��Al��= n��H2��=
n��H2��= ×
× mol=
mol=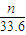 mol��
mol��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����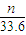 mol��
mol��
��2������������ϡ���ᣬFe��Al�����ᶼ��Ӧ�����������������������ʵ���=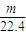 mol�����ݵ���ת���غ��֪��ת�Ƶ��ӵ����ʵ���=
mol�����ݵ���ת���غ��֪��ת�Ƶ��ӵ����ʵ���=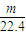 mol×2=
mol×2= mol��
mol��
Al���ɵ���������������������Ʒ�Ӧ���ɵ������������Fe�����ᷴӦ�������������Ϊ��m-n��L�����������ʵ���Ϊ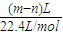 =
= mol�����ݵ���ת�ƿ�֪n��Fe��=n��H2��=
mol�����ݵ���ת�ƿ�֪n��Fe��=n��H2��= mol����m��Fe��=
mol����m��Fe��=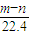 mol×56g/mol=2.5��m-n��g��
mol×56g/mol=2.5��m-n��g��
�ʴ�Ϊ��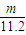 ��2.5��m-n��g��
��2.5��m-n��g��
�ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����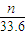 mol��
mol��
��3����2�����õ���Һ���������NaOH��Һ��������ת��Ϊƫ����������������������������պ����ù���Ϊ�������������������ԭ����������ǡ����ȣ�������������Ԫ�ص��������ڻ������Al����������ԭ�������Al����������������������Ԫ�ص���������Ϊ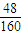 ×100%=30%��
×100%=30%��
�ʴ�Ϊ��30%��
���������⿼��������йؼ��㣬�Ѷ��еȣ����������Ӧ�ǽ���Ĺؼ���ע���غ�˼������ã�
����n=
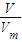 �������������ʵ������������ӷ���ʽ����Al�����ʵ�����
�������������ʵ������������ӷ���ʽ����Al�����ʵ�������2������n=
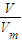 �������������ʵ��������ݵ���ת���غ����ת�Ƶ������ʵ�����
�������������ʵ��������ݵ���ת���غ����ת�Ƶ������ʵ���������������ϡ���ᣬFe��Al�����ᶼ��Ӧ����������Al���ɵ���������������������Ʒ�Ӧ���ɵ�����������ݴ˼���Fe�����ᷴӦ������������������ݵ���ת�ƿ�֪n��Fe��=n��H2�����ٸ���m=nM���㣻
��3����2�����õ���Һ���������NaOH��Һ��������ת��Ϊƫ����������������������������պ����ù���Ϊ�������������������ԭ����������ǡ����ȣ�������������Ԫ�ص��������ڻ������Al���������ݴ˽���������Ļ�ѧʽ����ԭ�������Al������������
����⣺��1�������������Ʋ���Ӧ����������������Һ��Ӧ����ƫ�����ƺ���������Ӧ���ӷ���ʽΪ��2Al+2OH-+2H2O=2AlO2-+3H2����
n L���������ʵ���=
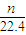 mol���ɷ���ʽ��֪n��Al��=
mol���ɷ���ʽ��֪n��Al��= n��H2��=
n��H2��= ×
×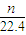 mol=
mol=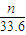 mol��
mol���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
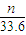 mol��
mol����2������������ϡ���ᣬFe��Al�����ᶼ��Ӧ�����������������������ʵ���=
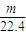 mol�����ݵ���ת���غ��֪��ת�Ƶ��ӵ����ʵ���=
mol�����ݵ���ת���غ��֪��ת�Ƶ��ӵ����ʵ���=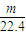 mol×2=
mol×2= mol��
mol��Al���ɵ���������������������Ʒ�Ӧ���ɵ������������Fe�����ᷴӦ�������������Ϊ��m-n��L�����������ʵ���Ϊ
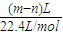 =
= mol�����ݵ���ת�ƿ�֪n��Fe��=n��H2��=
mol�����ݵ���ת�ƿ�֪n��Fe��=n��H2��= mol����m��Fe��=
mol����m��Fe��=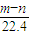 mol×56g/mol=2.5��m-n��g��
mol×56g/mol=2.5��m-n��g���ʴ�Ϊ��
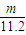 ��2.5��m-n��g��
��2.5��m-n��g���ʴ�Ϊ��2Al+2OH-+2H2O=2AlO2-+3H2����
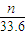 mol��
mol����3����2�����õ���Һ���������NaOH��Һ��������ת��Ϊƫ����������������������������պ����ù���Ϊ�������������������ԭ����������ǡ����ȣ�������������Ԫ�ص��������ڻ������Al����������ԭ�������Al����������������������Ԫ�ص���������Ϊ
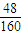 ×100%=30%��
×100%=30%���ʴ�Ϊ��30%��
���������⿼��������йؼ��㣬�Ѷ��еȣ����������Ӧ�ǽ���Ĺؼ���ע���غ�˼������ã�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ