��Ŀ����
��2009?�ɶ�һģ����֪��CH3CH2CH2CH3��g��+
O2��g��=4CO2��g��+5H2O��l����H=-2878kJ?mol-1����CH3��2CHCH3��g��+
O2��g��=4CO2��g��+5H2O��l����H=-2869kJ?mol-1����˵����ȷ���ǣ�������
| 13 |
| 2 |
| 13 |
| 2 |
������A�������Ȼ�ѧ����ʽ�еĶ�����ϵ����Ƚϣ�
B�����������Ľṹ�����������������������̼ԭ�Ӷ����Ե���������ԭ�����ӣ�
C����������Ȼ�ѧ����ʽ����ʽ����õ�CH3CH2CH2CH3ת��Ϊ��CH3��2CHCH3�������ߵ����жϣ�
D����������Ȼ�ѧ����ʽ��ʽ����õ��춡��ת��Ϊ��������Ȼ�ѧ����ʽ���������жϣ�
B�����������Ľṹ�����������������������̼ԭ�Ӷ����Ե���������ԭ�����ӣ�
C����������Ȼ�ѧ����ʽ����ʽ����õ�CH3CH2CH2CH3ת��Ϊ��CH3��2CHCH3�������ߵ����жϣ�
D����������Ȼ�ѧ����ʽ��ʽ����õ��춡��ת��Ϊ��������Ȼ�ѧ����ʽ���������жϣ�
����⣺A���Ȼ�ѧ����ʽ�����ֿ�ȼ���ʵ�ȼ���ȣ��Ƚ����ݱ�ɵõ������ʵ�����������ȼ��ʱ�ų����������춡��࣬��A����
B����������������춡����ͬ���칹�壬��������������������̼ԭ���γɵĻ�ѧ�����ǵ��������Է������ж�����ԭ�ӣ����ж���̼��������Զ�������̼�����ͬ����B����
C���Ȼ�ѧ����ʽ����õ�CH3CH2CH2CH3��g��=��CH3��2CHCH3��g����H=-9KJ?mol-1���˹�����һ�����ȹ��̣��������������������춡�飬��C��ȷ��
D���Ȼ�ѧ����ʽ����õ���CH3��2CHCH3��g���TCH3CH2CH2CH3��g����H=9KJ?mol-1���춡��ת��Ϊ������Ĺ�����һ�����ȹ��̣���D����
��ѡC��
B����������������춡����ͬ���칹�壬��������������������̼ԭ���γɵĻ�ѧ�����ǵ��������Է������ж�����ԭ�ӣ����ж���̼��������Զ�������̼�����ͬ����B����
C���Ȼ�ѧ����ʽ����õ�CH3CH2CH2CH3��g��=��CH3��2CHCH3��g����H=-9KJ?mol-1���˹�����һ�����ȹ��̣��������������������춡�飬��C��ȷ��
D���Ȼ�ѧ����ʽ����õ���CH3��2CHCH3��g���TCH3CH2CH2CH3��g����H=9KJ?mol-1���춡��ת��Ϊ������Ĺ�����һ�����ȹ��̣���D����
��ѡC��
���������⿼�����Ȼ�ѧ����ʽ��Ӧ�ã����ʵ������ߵͱȽϣ��л����ʷ��ӽṹ���жϵ�֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
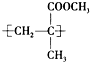 ��2009?�ɶ�һģ��һ���л������Ľṹ��ʽ��ͼ��ʾ�����й���������������ȷ���ǣ�������
��2009?�ɶ�һģ��һ���л������Ľṹ��ʽ��ͼ��ʾ�����й���������������ȷ���ǣ�������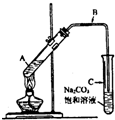 ��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о���
��2009?�ɶ�һģ��ij��ȤС��������ͼװ�ã�ȡ��ͬŨ�ȵ������� 3mL ��ˮ�Ҵ��� 2mL ������ֱ������ȡ�����������о��� CH3COOCH2CH3+H2O
CH3COOCH2CH3+H2O