��Ŀ����
��ƺ�����ǹŴ��Ͷ�������ǻ۽ᾧ���ƺʹ�Ҳ���ճ������г������л����1����������ڳ�ȥˮ���е�ˮ�����ɷ���Ҫ��CaCO3��Mg��OH��2��д��������Mg��OH��2��Ӧ�����ӷ���ʽ______��ʳ���г�����KIO3�����⣬Ϊ��֤ʳ���е�KIO3������ʳ���м�����ᣬ�ټ���KI�͵�����Һ����Ӧ�����ӷ���ʽΪ______��
��2�������ƵĹ����������еĵ�����ø�����������������ǣ�д����ѧ����ʽ______
���𰸡���������1���������̼���η�Ӧ�Ĺ�����д��ѧ����ʽ������KIO3��KI�������ܷ������з�Ӧ�Լ�I2��ʹ���۱�����
��2������������Ϣ�����ѧ֪ʶ�ҳ���Ӧ�ķ�Ӧ��������P��Ӧ������Ȼ���ϻ�ѧ����ʽ����дҪ��������𣻣�3������Ǧ���ؽ������������ж���
����⣺��1��������ˮ����CaCO3��Ӧ���ɴ���ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��2CH3COOH+CaCO3�T��CH3COO��2Ca+CO2��+H2O����KIO3��KI�������ܷ������з�Ӧ��KIO3+5KI+6CH3COOH=6CH3COOK+3H2O+3I2���⻯�ر�����������ر���ԭ�������ṩ�ȶ����Ի������ʴ�Ϊ��2CH3COOH+CaCO3�T2CH3COO-+Ca2++CO2��+H2O��IO3-+5I-+6CH3COOH=6CH3COO-+3H2O+3I2��
��2������������Ϣ��֪��Ӧ��Ϊ��ά�غ�ˮ��������Ϊ�����ǣ�øΪ��������Ӧ����ʽΪ����C6H10O5��n�����ۣ�+H2O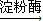 nC6H12O6�������ǣ����ʴ�Ϊ����C6H10O5��n�����ۣ�+nH2O
nC6H12O6�������ǣ����ʴ�Ϊ����C6H10O5��n�����ۣ�+nH2O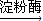 nC6H12O6�������ǣ���
nC6H12O6�������ǣ���
��3����Ǧ���ؽ�����Pb2+�ж����ʴ�Ϊ�������У�Ǧ���ؽ�����Pb2+�������ж���
������������Ҫ��������������ʣ��ѶȲ���ֻҪ���ջ���֪ʶ������ɣ�
��2������������Ϣ�����ѧ֪ʶ�ҳ���Ӧ�ķ�Ӧ��������P��Ӧ������Ȼ���ϻ�ѧ����ʽ����дҪ��������𣻣�3������Ǧ���ؽ������������ж���
����⣺��1��������ˮ����CaCO3��Ӧ���ɴ���ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��2CH3COOH+CaCO3�T��CH3COO��2Ca+CO2��+H2O����KIO3��KI�������ܷ������з�Ӧ��KIO3+5KI+6CH3COOH=6CH3COOK+3H2O+3I2���⻯�ر�����������ر���ԭ�������ṩ�ȶ����Ի������ʴ�Ϊ��2CH3COOH+CaCO3�T2CH3COO-+Ca2++CO2��+H2O��IO3-+5I-+6CH3COOH=6CH3COO-+3H2O+3I2��
��2������������Ϣ��֪��Ӧ��Ϊ��ά�غ�ˮ��������Ϊ�����ǣ�øΪ��������Ӧ����ʽΪ����C6H10O5��n�����ۣ�+H2O
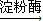 nC6H12O6�������ǣ����ʴ�Ϊ����C6H10O5��n�����ۣ�+nH2O
nC6H12O6�������ǣ����ʴ�Ϊ����C6H10O5��n�����ۣ�+nH2O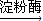 nC6H12O6�������ǣ���
nC6H12O6�������ǣ�����3����Ǧ���ؽ�����Pb2+�ж����ʴ�Ϊ�������У�Ǧ���ؽ�����Pb2+�������ж���
������������Ҫ��������������ʣ��ѶȲ���ֻҪ���ջ���֪ʶ������ɣ�

��ϰ��ϵ�д�
�����Ŀ