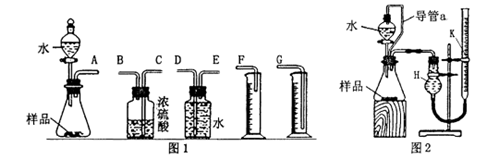
��Ŀ����
��15�֣�������������������з�������Ҫ�����á�
��1���ơ��������dz����Ľ�����
�������ֽ���Ԫ���У����γ����������������� ������Ԫ���γɵ������������ڼ������������ �֡�
����ʢ����������Һ���Թ��еμӰ�ˮ����Ӧ�����ӷ���ʽΪ ��
�õ��Ľ�״��������ҽ��������θ����࣬���õ�ԭ�������ӷ���ʽ��ʾΪ ��
����ʢ���Ȼ������Ȼ��������Ȼ�ͭ�����Һ���ձ��м������ۺ�ͭ�ۣ���Ӧ������ʣ���������ͭ����������Һ��һ�����ڵ�������Ϊ �����ܴ��ڵ�������Ϊ ��һ�������ڵ�������Ϊ ��
���������������ȷ�Ӧ���������������ﷴӦ�������������������Ӹֹ죬������������������Ӧ�Ļ�ѧ����ʽΪ���������������������������� ����Ҫ�õ�1mol����������Ҫ������������������g��
��2����ҵ�ϣ��ڵ�¯����̿�ۻ�ԭ����������Ƶù��һ����̼�������¯���������̿�ۺ�30g�������裬ͨ���Ӧ�����ɵ�һ����̼�ڱ���µ����Ϊ ��
��1���ơ��������dz����Ľ�����
�������ֽ���Ԫ���У����γ����������������� ������Ԫ���γɵ������������ڼ������������ �֡�
����ʢ����������Һ���Թ��еμӰ�ˮ����Ӧ�����ӷ���ʽΪ ��
�õ��Ľ�״��������ҽ��������θ����࣬���õ�ԭ�������ӷ���ʽ��ʾΪ ��
����ʢ���Ȼ������Ȼ��������Ȼ�ͭ�����Һ���ձ��м������ۺ�ͭ�ۣ���Ӧ������ʣ���������ͭ����������Һ��һ�����ڵ�������Ϊ �����ܴ��ڵ�������Ϊ ��һ�������ڵ�������Ϊ ��
���������������ȷ�Ӧ���������������ﷴӦ�������������������Ӹֹ죬������������������Ӧ�Ļ�ѧ����ʽΪ���������������������������� ����Ҫ�õ�1mol����������Ҫ������������������g��
��2����ҵ�ϣ��ڵ�¯����̿�ۻ�ԭ����������Ƶù��һ����̼�������¯���������̿�ۺ�30g�������裬ͨ���Ӧ�����ɵ�һ����̼�ڱ���µ����Ϊ ��
��1���� Fe������1�֣���3��1�֣�
�� Al3+ + 3NH3.H2O = Al(OH)3��+ 3NH4+��2�֣���Al(OH)3 + 3H+ = Al3+ + 3H2O��2�֣�
��Fe2+��1�֣���Cu 2����1�֣���Fe3+��1�֣�
��2Al + Fe2O3 = Al2O3+ 2Fe��2�֣� ��27��2�֣�
��2��22.4L��2�֣�
�� Al3+ + 3NH3.H2O = Al(OH)3��+ 3NH4+��2�֣���Al(OH)3 + 3H+ = Al3+ + 3H2O��2�֣�
��Fe2+��1�֣���Cu 2����1�֣���Fe3+��1�֣�
��2Al + Fe2O3 = Al2O3+ 2Fe��2�֣� ��27��2�֣�
��2��22.4L��2�֣�
�����������1���ơ��������dz����Ľ�����
�������ֽ���Ԫ���У����γ�������������������������Ԫ���γɵ������������ڼ������������3�֡�
����ʢ����������Һ���Թ��еμӰ�ˮ����Ӧ�����ӷ���ʽΪAl3+ + 3NH3.H2O = Al(OH)3��+ 3NH4+��Al(OH)3�������Ӧ��
�õ��Ľ�״��������ҽ��������θ����࣬θ�������ᣬ���õ�ԭ�������ӷ���ʽ��ʾΪAl(OH)3 + 3H+ = Al3+ + 3H2O��
����ʢ���Ȼ������Ȼ��������Ȼ�ͭ�����Һ���ձ��м������ۺ�ͭ�ۣ���Ӧ������ʣ���������ͭ����������Һ��һ�����ڵ�������ΪFe2+�����ܴ��ڵ�������ΪCu 2����һ�������ڵ�������ΪFe3+������ͭʣ�࣬��һ����������������
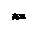 ���������������ȷ�Ӧ���������������ﷴӦ�������������������Ӹֹ죬������������������Ӧ�Ļ�ѧ����ʽΪ2Al + Fe2O3 = Al2O3+ 2Fe��Ҫ�õ�1mol���������ɶ�����ϵ�ɵ���Ҫ�������ʵ�����1mol������������27g��
���������������ȷ�Ӧ���������������ﷴӦ�������������������Ӹֹ죬������������������Ӧ�Ļ�ѧ����ʽΪ2Al + Fe2O3 = Al2O3+ 2Fe��Ҫ�õ�1mol���������ɶ�����ϵ�ɵ���Ҫ�������ʵ�����1mol������������27g����2����ҵ�ϣ��ڵ�¯����̿�ۻ�ԭ����������Ƶù��һ����̼��
SiO2 + 2C = 2CO�� + Si
60 2��22.4
30 x
X=22.4
�������¯���������̿�ۺ�30g�������裬ͨ���Ӧ�����ɵ�һ����̼�ڱ���µ����Ϊ22.4L��
���������⿼��������֮��ķ�Ӧ�����⣬Ҫ��ѧ����Ϥ���������ʼ�ķ�Ӧ�����ö�����ϵ���м��㡣

��ϰ��ϵ�д�
�����Ŀ