��Ŀ����
��11�֣� A��B��C��D��E��F����Ԫ�أ����ǵĺ˵������С��18����ԭ��������������֪��A��C��F����ԭ�ӵ�����㹲��11�����ӣ���������Ԫ�ص�����������ˮ����֮���������ܷ�Ӧ���������κ�ˮ��11.5g A����ǡ����100mL 5mol/L��������ȫ��Ӧ����Ӧ����Һ�����ԡ�DԪ��ԭ�ӵ������������ȴ�����������4����EԪ��ԭ�ӵĴ�����������������������3������ش��������⣺
��1��D��Ԫ�ط��� ��E������������ˮ����ķ���ʽ ��
��2������BԪ�ص�ԭ�ӽṹʾ��ͼ ��
��3��������FԪ�ص�һ��ԭ�ӵ�������Ϊ35��д����ԭ�ӷ��� ����ԭ�Ӻ��ڵ��������� ��
��4��A��F��C��F��A��CԪ������������ˮ���ﷴӦ�����ӷ���ʽ����
�� �� �� ��
��1��D��Ԫ�ط��� ��E������������ˮ����ķ���ʽ ��
��2������BԪ�ص�ԭ�ӽṹʾ��ͼ ��
��3��������FԪ�ص�һ��ԭ�ӵ�������Ϊ35��д����ԭ�ӷ��� ����ԭ�Ӻ��ڵ��������� ��
��4��A��F��C��F��A��CԪ������������ˮ���ﷴӦ�����ӷ���ʽ����
�� �� �� ��
��11�֣���1��Si H3PO4 ��2��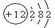
��3��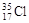 18
18
��4��H++OH- = H2O Al(OH)3+3H+=Al3++3H2O Al(OH)3+OH- = AlO2-+2H2O
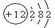
��3��
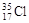 18
18��4��H++OH- = H2O Al(OH)3+3H+=Al3++3H2O Al(OH)3+OH- = AlO2-+2H2O
����Ԫ�ص�����������ˮ����֮���������ܷ�Ӧ���������κ�ˮ��˵��һ��������Ԫ�ء�����ԭ��������С˳���֪��C������A�ǵ�IAԪ�أ�����F����Ԫ�ء�11.5g A����ǡ����100mL 5mol/L��������ȫ��Ӧ����Ӧ����Һ�����ԣ����A�����ԭ��������11.5��0.5��23������A����Ԫ�أ���B��Mg��DԪ��ԭ�ӵ������������ȴ�����������4������D�ǹ衣EԪ��ԭ�ӵĴ�����������������������3������E��P��

��ϰ��ϵ�д�
�����Ŀ
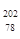 Pt��
Pt��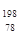 Pt��˵����ȷ����
Pt��˵����ȷ����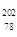 Pt��
Pt��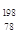 Pt����������ͬ
Pt����������ͬ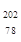 Pt��
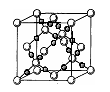
Pt��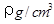 ��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ
��NAΪ�����ӵ����������ڵ�����Cs+�ĺ˼��Ϊa cm����ͼ��ʾ����CsCl��Ħ���������Ա�ʾΪ 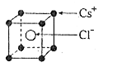
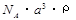 g/mol B��
g/mol B��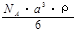 g/mol
g/mol 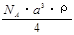 g/mol D��
g/mol D��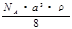 g/mol
g/mol