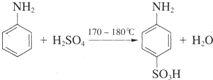
��Ŀ����
��2010?���ն�ģ����NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ�������
������A��pH=7��NH4Cl��NH3?H2O�����Һ�У�OH-Ũ��Ϊ10-7mol/L����Һ��������Ŀ������Ũ�ȡ���Һ����йأ�
B����״���£�NO��O2��11.2L���������ʵ���Ϊ0.5mol����Ϸ�����Ӧ2NO+O2=2NO2�����ɶ�������0.5mol��ʣ������0.25mol�����û���������ƽ��2NO2?N2O4���������û����������ʵ���С��0.75mol��
C��ÿ��CnH2n�����к���1��C=C˫��������n=
����14gCnH2n�����ʵ������ٸ���N=nNA����̼̼˫������
D������n=
�����������ʵ����������������������������Һ��Ӧ����ʽ���й������㣬����������ԭ��Ӧ�е���ת�ƽ��N=nNA����ת�Ƶ���������
B����״���£�NO��O2��11.2L���������ʵ���Ϊ0.5mol����Ϸ�����Ӧ2NO+O2=2NO2�����ɶ�������0.5mol��ʣ������0.25mol�����û���������ƽ��2NO2?N2O4���������û����������ʵ���С��0.75mol��
C��ÿ��CnH2n�����к���1��C=C˫��������n=
| m |
| M |
D������n=
| m |
| M |
����⣺A��pH=7��NH4Cl��NH3?H2O�����Һ�У�OH-Ũ��Ϊ10-7mol/L����Һ��������Ŀ������Ũ�ȡ���Һ����йأ���Һ���δ���ߣ�������������������Ŀ����A����
B����״���£�NO��O2��11.2L���������ʵ���Ϊ0.5mol����Ϸ�����Ӧ2NO+O2=2NO2�����ɶ�������0.5mol��ʣ������0.25mol�����û���������ƽ��2NO2?N2O4���������û����������ʵ���С��0.75mol�����û������ķ�������ΪС��0.75 NA����B����
C��14gCnH2n�����ʵ���Ϊ
=
mol��ÿ��CnH2n�����к���1��C=C˫�������ԣ�14gCnH2n�к��е�̼̼˫����Ϊ
����C��ȷ��
D��2.7g�����ʵ���Ϊ0.1mol��100mLŨ��Ϊ2 mol?L-1�����������������Һ���е��Ȼ��⡢�������Ƶ����ʵ���Ϊ
0.2mol����2Al+2NaOH+2H2O�T2NaAlO2+3H2����֪�������������Ʒ�Ӧ������ȫ��Ӧ��ת�Ƶ�����Ϊ0.1mol��3��NAmol-1=0.3NA����2Al+6HCl�T2AlCl3+3H2����֪����㣬������������0.1mol��ת�Ƶ�����Ϊ0.1mol��2��NAmol-1=0.2NA����D����
��ѡ��C��
B����״���£�NO��O2��11.2L���������ʵ���Ϊ0.5mol����Ϸ�����Ӧ2NO+O2=2NO2�����ɶ�������0.5mol��ʣ������0.25mol�����û���������ƽ��2NO2?N2O4���������û����������ʵ���С��0.75mol�����û������ķ�������ΪС��0.75 NA����B����
C��14gCnH2n�����ʵ���Ϊ
| 14g |
| 14ng/mol |
| 1 |
| n |
| NA |
| n |
D��2.7g�����ʵ���Ϊ0.1mol��100mLŨ��Ϊ2 mol?L-1�����������������Һ���е��Ȼ��⡢�������Ƶ����ʵ���Ϊ
0.2mol����2Al+2NaOH+2H2O�T2NaAlO2+3H2����֪�������������Ʒ�Ӧ������ȫ��Ӧ��ת�Ƶ�����Ϊ0.1mol��3��NAmol-1=0.3NA����2Al+6HCl�T2AlCl3+3H2����֪����㣬������������0.1mol��ת�Ƶ�����Ϊ0.1mol��2��NAmol-1=0.2NA����D����
��ѡ��C��
���������鳣�û�ѧ�����йؼ��㣬�Ѷ��еȣ�ע��Bѡ���д���ƽ��2NO2?N2O4��Ӱ�������Ŀ������Ӱ��ԭ����Ŀ��

��ϰ��ϵ�д�
�����Ŀ