��Ŀ����
(12 ��)�Ե�ʯ��[��Ҫ�ɷ���Ca(OH)2����SiO2��Al2O3�Լ�������������]Ϊԭ���������������̼��Ƶ��������£�
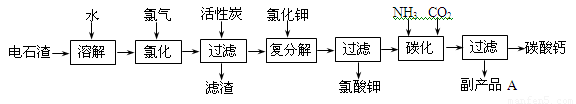
�ش��������⣺
��1����ʯ������ˮ�γɵ�ʯ����ʱ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
_____________________________________��__________________________________��
��2���Ȼ����̵��¶ȿ�����75��80�棬�ù�����Ҫ��Ӧ�Ļ�ѧ����ʽΪ��
________________________________________________________________________��
��3���������м������̿��������_____________________________________________��
��4����������̼����Ӧ�����ӷ���ʽΪ_________________________________________��
��5������ƷA�Ļ�ѧʽΪ________________��
(12��)
��1��Ca(OH)2 + SiO2 = CaSiO3 + H2O ��2�֣� Ca(OH)2 + Al2O3 = Ca(AlO2)2 + H2O��2�֣�
��2��6Cl2 + 6Ca(OH)2 Ca(ClO3)2 + 5CaCl2
+ 6H2O��2�֣�
Ca(ClO3)2 + 5CaCl2
+ 6H2O��2�֣�
��3��������������ֹ�ں���ʵ���������ݳ���Ⱦ���� ��2�֣�
��4��Ca2+ + 2NH3 + CO2 + H2O = CaCO3�� + 2NH4+��2�֣�
��5��NH4Cl�������������𰸣���2�֣�
��������
�����������1��SiO2��Al2O3�������[Ca(OH)2]������Ӧ������ʽΪ��Ca(OH)2 + SiO2 = CaSiO3 + H2O Ca(OH)2 + Al2O3 = Ca(AlO2)2 + H2O��
��2���Ȼ������Ƿ�Ӧ�������Ca(OH)2����ѧ����ʽΪ6Cl2
+ 6Ca(OH)2 Ca(ClO3)2 + 5CaCl2
+ 6H2O��
Ca(ClO3)2 + 5CaCl2
+ 6H2O��
��3�������ж����һ���̿�����������ã��ʼ������̿��������������������ֹ�ں���ʵ���������ݳ���Ⱦ������
���㣺��ѧʵ��
�������������й�ʵ�鷽������ƺ����۵Ŀ��飬Ҫ��ѧ����Ϥ��ʵ������ݼ�ԭ�����ܹ�����ͬѧ�ǽ��з������⡢��������������

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�