��Ŀ����
����Ŀ����ϩ��ʯ�ͻ�ѧ��ҵ����Ҫ����ԭ�ϣ��ҹ���ѧ�����ü״�ת���Ʊ�ϩ��Ӧ�������£�
3CH3OH +H3AlO6 ��3![]() +
+![]() +3H2O
+3H2O
3![]() +
+![]() ��H3AlO6 + 3C������H2
��H3AlO6 + 3C������H2
3C������H2 �� CH2=CHCH3
���������������
A.�״�ת���Ʊ�ϩ��Ӧ�ķ���ʽΪ3CH3OH��CH2=CHCH3+3H2O
B.�״�ת���Ʊ�ϩ��Ӧ�Ĺ�����H3AlO6������
C.1.4 g C������H2�����ĵ��ӵ����ʵ���Ϊ1 mol
D.��̼������![]() �ĵ���ʽΪ
�ĵ���ʽΪ![]()
���𰸡�C
��������
���������������ʽ��Ӽ��ü״�ת���Ʊ�ϩ��Ӧ�ķ���ʽ��ѡ��A��ȷ���ڷ�Ӧǰ��H3AlO6����Ӧ����������H3AlO6�����������䣬���ϴ����Ķ��壬ѡ��B��ȷ����Ӧǰ��ԭ���غ㣬����Ҳ�غ㣬 1.4 g C������H2�����ĵ��ӵ����ʵ���Ϊ0.8 mol������ѡ��C����̼�������Ǽ�ʧȥһ�������γɵ������ӣ�ѡ��D��ȷ��

��ϰ��ϵ�д�
�����Ŀ
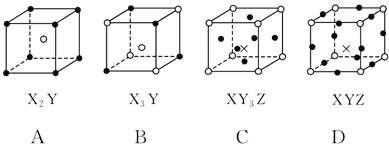
����Ŀ���ס��������ֳ����Ļ����X��Y��Z�����ֳ����ĵ��ʡ��±����и�������֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾת�����ǣ� ��

ѡ�� | X | Y | Z | �� | �� |
A | C | H2 | O2 | H2O | CO |
B | Zn | Fe | Cl2 | FeCl2 | ZnCl2 |
C | Mg | C | O2 | CO2 | MgO |
D | H2 | Si | Cl2 | SiCl4 | HCl |
A.AB.BC.CD.D