��Ŀ����
��1��������������� ��
�� ��CH3CH2CH2CH3 ��
��CH3CH2CH2CH3 ��
�����������ǣ�����ţ���ͬ��
��2������5���л����������������CH3COOCH2CH3�������ӣ� ������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
����������NaHCO3��Һ��Ӧ�������ݵ���
����������FeCl3��Һ������ɫ��Ӧ����
 ��
��
�������ܷ���ˮ�ⷴӦ����
��д����ȩ��������Һ����������Ӧ�Ļ�ѧ����ʽ
��3������ת����ϵ�У��л���B�ķ���ʽ��C3H6��C��һ�ָ߷��Ӳ��ϣ�
��ش��������⣺
��C��D�Ľṹ��ʽ�ֱ���
 ��
��
��A����B�Ļ�ѧ��Ӧ������
��A�к��еĹ����ŵ�������
��A��E������Ӧ�Ļ�ѧ����ʽ��
 ��
�� ��CH3CH2CH2CH3 ��
��CH3CH2CH2CH3 ��
�����������ǣ�����ţ���ͬ��
�٢�
�٢�
����ڻ�Ϊͬϵ�������
��
����ۻ�Ϊͬ���칹�������
��
����ϵͳ����������������������Ϊ2-������
2-������
����2������5���л����������������CH3COOCH2CH3�������ӣ�
 ������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH��������������NaHCO3��Һ��Ӧ�������ݵ���
CH3COOH
CH3COOH
������������FeCl3��Һ������ɫ��Ӧ����


�������ܷ���ˮ�ⷴӦ����
����������CH3COOCH2CH3����
����������CH3COOCH2CH3����
����д����ȩ��������Һ����������Ӧ�Ļ�ѧ����ʽ
CH3CHO+2Ag��NH3��2OH
CH3COONH4+H2O+2Ag��+3NH3
| �� |
CH3CHO+2Ag��NH3��2OH
CH3COONH4+H2O+2Ag��+3NH3
��| �� |
��3������ת����ϵ�У��л���B�ķ���ʽ��C3H6��C��һ�ָ߷��Ӳ��ϣ�

��ش��������⣺
��C��D�Ľṹ��ʽ�ֱ���


CH3CH2CHO
CH3CH2CHO
����A����B�Ļ�ѧ��Ӧ������
��ȥ��Ӧ
��ȥ��Ӧ
����A�к��еĹ����ŵ�������
�ǻ�
�ǻ�
����A��E������Ӧ�Ļ�ѧ����ʽ��
CH3CH2COOH+CH3CH2CH2OH
CH3CH2COOCH2CH2CH3+H2O
| Ũ���� |
| �� |
CH3CH2COOH+CH3CH2CH2OH
CH3CH2COOCH2CH2CH3+H2O
��| Ũ���� |
| �� |
��������1�����������ͨʽΪCnH2n+2�������в����ڲ����ͼ��뱽����
����ʽ��ͬ���ṹ��ͬ�Ļ����ﻥ��ͬ���칹�壻
�ṹ���ƣ���������γ�1�������ɸ�CH2ԭ�������ʣ���Ϊͬϵ�
����ϵͳ����������������
��2��������NaHCO3��Һ��Ӧ�������ݣ����л�������к����Ȼ���-COOH����
������FeCl3��Һ������ɫ��Ӧ�����л�������к��з��ǻ���-OHֱ���뱽����������
���������ʵȿ��Է���ˮ�ⷴӦ����ˮ�ⷴӦ��
����ȩ��������Һ����������Ӧ����������李�����ˮ��
��3����ת����ϵͼ��֪���л���A��Ũ���ᡢ��������������B��C3H6������Cu������������������������Ӧ����D��D���Է���������Ӧ����DΪ��ȩ��AΪ1-������BΪ��ϩ��C��һ�ָ߷��Ӳ��ϣ�CΪ�۱�ϩ��EΪ���ᣮ
����ʽ��ͬ���ṹ��ͬ�Ļ����ﻥ��ͬ���칹�壻
�ṹ���ƣ���������γ�1�������ɸ�CH2ԭ�������ʣ���Ϊͬϵ�
����ϵͳ����������������
��2��������NaHCO3��Һ��Ӧ�������ݣ����л�������к����Ȼ���-COOH����
������FeCl3��Һ������ɫ��Ӧ�����л�������к��з��ǻ���-OHֱ���뱽����������
���������ʵȿ��Է���ˮ�ⷴӦ����ˮ�ⷴӦ��
����ȩ��������Һ����������Ӧ����������李�����ˮ��
��3����ת����ϵͼ��֪���л���A��Ũ���ᡢ��������������B��C3H6������Cu������������������������Ӧ����D��D���Է���������Ӧ����DΪ��ȩ��AΪ1-������BΪ��ϩ��C��һ�ָ߷��Ӳ��ϣ�CΪ�۱�ϩ��EΪ���ᣮ
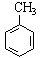
����⣺��1������ �� ��CH3CH2CH2CH3���������������߷���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壻
�� ��CH3CH2CH2CH3���������������߷���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壻
�� �� ��
�� �� ����1���������ṹ���ƣ��γ�1��CH2ԭ���ţ���Ϊͬϵ�
����1���������ṹ���ƣ��γ�1��CH2ԭ���ţ���Ϊͬϵ�
�� ��ϵͳ����������Ϊ��2-�����飬
��ϵͳ����������Ϊ��2-�����飬
�ʴ�Ϊ���٢ۣ��ܣ��٣�2-�����飻
��2�������Ậ��-COOH������NaHCO3��Һ��Ӧ���ɶ�����̼��
�ʴ�Ϊ��CH3COOH��
�� ���з��ǻ�������FeCl3��Һ������ʹ��Һ����ɫ��
���з��ǻ�������FeCl3��Һ������ʹ��Һ����ɫ��
�ʴ�Ϊ�� ��
��
������������CH3COOCH2CH3���к������������Է���ˮ�⣬
�ʴ�Ϊ������������CH3COOCH2CH3����
����ȩ��������Һ����������Ӧ����������李���������ˮ����Ӧ����ʽΪCH3CHO+2Ag��NH3��2OH
CH3COONH4+H2O+2Ag��+3NH3��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
CH3COONH4+H2O+2Ag��+3NH3��
��3����ת����ϵͼ��֪���л���A��Ũ���ᡢ��������������B��C3H6������Cu������������������������Ӧ����D��D���Է���������Ӧ����DΪ��ȩ��AΪ1-������BΪ��ϩ��C��һ�ָ߷��Ӳ��ϣ�CΪ�۱�ϩ��EΪ���ᣮ
��CΪ�۱�ϩ���ṹ��ʽΪ ��DΪ��ȩ���ṹ��ʽΪCH3CH2CHO��
��DΪ��ȩ���ṹ��ʽΪCH3CH2CHO��
�ʴ�Ϊ�� ��CH3CH2CHO��
��CH3CH2CHO��
��AΪ1-������BΪ��ϩ��1-����������ȥ��Ӧ���ɱ�ϩ��
�ʴ�Ϊ����ȥ��Ӧ��
��AΪ1-������A�к��еĹ��������ǻ����ʴ�Ϊ���ǻ���
��AΪ1-������EΪ���ᣬA��E������Ӧ�Ļ�ѧ����ʽ��CH3CH2COOH+CH3CH2CH2OH
CH3CH2COOCH2CH2CH3+H2O��
�ʴ�Ϊ��CH3CH2COOH+CH3CH2CH2OH
CH3CH2COOCH2CH2CH3+H2O��
 �� ��CH3CH2CH2CH3���������������߷���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壻
�� ��CH3CH2CH2CH3���������������߷���ʽ��ͬ���ṹ��ͬ����Ϊͬ���칹�壻��
 �� ��
�� �� ����1���������ṹ���ƣ��γ�1��CH2ԭ���ţ���Ϊͬϵ�
����1���������ṹ���ƣ��γ�1��CH2ԭ���ţ���Ϊͬϵ���
 ��ϵͳ����������Ϊ��2-�����飬
��ϵͳ����������Ϊ��2-�����飬�ʴ�Ϊ���٢ۣ��ܣ��٣�2-�����飻
��2�������Ậ��-COOH������NaHCO3��Һ��Ӧ���ɶ�����̼��
�ʴ�Ϊ��CH3COOH��
��
 ���з��ǻ�������FeCl3��Һ������ʹ��Һ����ɫ��
���з��ǻ�������FeCl3��Һ������ʹ��Һ����ɫ���ʴ�Ϊ��
 ��
��������������CH3COOCH2CH3���к������������Է���ˮ�⣬
�ʴ�Ϊ������������CH3COOCH2CH3����
����ȩ��������Һ����������Ӧ����������李���������ˮ����Ӧ����ʽΪCH3CHO+2Ag��NH3��2OH
| �� |
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH
| �� |
��3����ת����ϵͼ��֪���л���A��Ũ���ᡢ��������������B��C3H6������Cu������������������������Ӧ����D��D���Է���������Ӧ����DΪ��ȩ��AΪ1-������BΪ��ϩ��C��һ�ָ߷��Ӳ��ϣ�CΪ�۱�ϩ��EΪ���ᣮ
��CΪ�۱�ϩ���ṹ��ʽΪ
 ��DΪ��ȩ���ṹ��ʽΪCH3CH2CHO��
��DΪ��ȩ���ṹ��ʽΪCH3CH2CHO���ʴ�Ϊ��
 ��CH3CH2CHO��
��CH3CH2CHO����AΪ1-������BΪ��ϩ��1-����������ȥ��Ӧ���ɱ�ϩ��
�ʴ�Ϊ����ȥ��Ӧ��
��AΪ1-������A�к��еĹ��������ǻ����ʴ�Ϊ���ǻ���
��AΪ1-������EΪ���ᣬA��E������Ӧ�Ļ�ѧ����ʽ��CH3CH2COOH+CH3CH2CH2OH
| Ũ���� |
| �� |
�ʴ�Ϊ��CH3CH2COOH+CH3CH2CH2OH
| Ũ���� |
| �� |
���������⿼��ͬϵ�ͬ���칹�塢ϵͳ�������������л�����������л���ͼ�ƶϵȣ��Ѷ��еȣ����ճ����л�����������ƶϵĹؼ���ע�����֪ʶ�����գ�

��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�����Ŀ
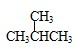



 ��
�� ��CH3CH2CH2CH3 ��
��CH3CH2CH2CH3 ��
 ������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����
������ȩ��CH3CHO�������ᣨCH3COOH�����Ҵ���CH3CH2OH����