��Ŀ����
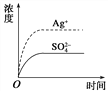
����Ŀ����֪Ag2SO4��KspΪ2.0��10��5 mol3��L��3��������Ag2SO4��������100 mLˮ�����պñ��ͣ��ù�����Ag�� ��SO42��Ũ����ʱ��仯��ϵ����ͼ[����Ag2SO4��Һ��c(Ag��)=0.034 molL��1]����t1ʱ����������ϵ�м���100 mL 0.020 molL��1 Na2SO4��Һ������ʾ��ͼ�У�����ȷ��ʾt1ʱ�̺�Ag�� ��SO42��Ũ����ʱ��仯��ϵ����
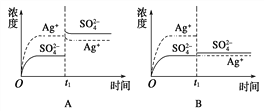

A. A B. B C. C D. D
���𰸡�B
��������
Ag2SO4�պ�Ϊ100ml�ı�����Һ����Ϊc(Ag+)=0.034molL-1����c(SO42-)=0.017molL-1��������100mL 0.020molL-1Na2SO4��Һ��c(SO42-)=![]() =0.0185molL-1��c(Ag+)=0.017molL-1����û�г������������Ϻ�������Ũ��Ϊԭ����һ�룬���������Ũ���������ɴ˿��Կ���ӦΪBͼ��ʱQ=c(SO42-)c2(Ag+)=0.0185��(0.017)2=5.346��10-6��Ksp=2.0��10-3�������Һ��û�г�����������ѡB��
=0.0185molL-1��c(Ag+)=0.017molL-1����û�г������������Ϻ�������Ũ��Ϊԭ����һ�룬���������Ũ���������ɴ˿��Կ���ӦΪBͼ��ʱQ=c(SO42-)c2(Ag+)=0.0185��(0.017)2=5.346��10-6��Ksp=2.0��10-3�������Һ��û�г�����������ѡB��

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
