��Ŀ����
д�����и��л���Ľṹ��ʽ��
��1��ij��1mol��2mol HCl��ȫ�ӳɣ����ɵ��ȴ�������������4mol������Ӧ��������Ľṹ��ʽΪ______________��
��2��ij����A�������ܶ�����ͬ״���������ܶȵ�64�������ⶨ��֪A�����й���6��������A��������ϩ���������ӳɵIJ��A�Ľṹ��ʽΪ___________ ;
��3�� 0.2 molij��A����������ȫȼ�պ�����CO2��H2O��1.2 mol��
������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ_________________________��
�� ����A��ʹ��ˮ��ɫ����A�ڴ��������¿�����H2 �����ӳɷ�Ӧ����ӳɲ�������к���4�����������A�ķ��������е�̼ԭ�Ӳ����ܴ���ͬһƽ���ϣ�����A���ܵĽṹ��ʽΪ___________��______________��
��1��ij��1mol��2mol HCl��ȫ�ӳɣ����ɵ��ȴ�������������4mol������Ӧ��������Ľṹ��ʽΪ______________��
��2��ij����A�������ܶ�����ͬ״���������ܶȵ�64�������ⶨ��֪A�����й���6��������A��������ϩ���������ӳɵIJ��A�Ľṹ��ʽΪ___________ ;
��3�� 0.2 molij��A����������ȫȼ�պ�����CO2��H2O��1.2 mol��
������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ_________________________��
�� ����A��ʹ��ˮ��ɫ����A�ڴ��������¿�����H2 �����ӳɷ�Ӧ����ӳɲ�������к���4�����������A�ķ��������е�̼ԭ�Ӳ����ܴ���ͬһƽ���ϣ�����A���ܵĽṹ��ʽΪ___________��______________��
��1��
��2��(CH3)3CCH2C(CH3)3��
��3���� �� ��CH2��CHC(CH3)3��CH2��C(CH3)CH(CH3)2
�� ��CH2��CHC(CH3)3��CH2��C(CH3)CH(CH3)2

��2��(CH3)3CCH2C(CH3)3��
��3����
 �� ��CH2��CHC(CH3)3��CH2��C(CH3)CH(CH3)2
�� ��CH2��CHC(CH3)3��CH2��C(CH3)CH(CH3)2 
��ϰ��ϵ�д�
�����Ŀ
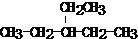


 ���������ŵ�������
���������ŵ�������
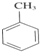 +3HNO3
+3HNO3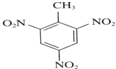 +3H2O��
+3H2O��