��Ŀ����
( 30�� )��1��3.6��H2O�����ʵ����� ������ ��H2O������ molH.
��2����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������֮��Ϊ���� �����ʵ���֮��Ϊ�� �� ��ԭ������֮��Ϊ���� ��������֮��Ϊ���� ��
��3���ڱ�״���£�22g CO2���������� L ���� g N2������ͬ�ķ�������
��4��3.01��1023��OH�������ʵ���Ϊ ������Ϊ ���������ӵ����ʵ���Ϊ �����е��ӵ����ʵ���Ϊ ����ЩOH���� molNH3��������ͬ���� g Na+���е���������ͬ��
�����е��ӵ����ʵ���Ϊ ����ЩOH���� molNH3��������ͬ���� g Na+���е���������ͬ��
��2����ͬ��ͬѹ�£�ͬ����ļ��飨CH4���Ͷ�����̼������֮��Ϊ���� �����ʵ���֮��Ϊ�� �� ��ԭ������֮��Ϊ���� ��������֮��Ϊ���� ��
��3���ڱ�״���£�22g CO2���������� L ���� g N2������ͬ�ķ�������
��4��3.01��1023��OH�������ʵ���Ϊ ������Ϊ ���������ӵ����ʵ���Ϊ
 �����е��ӵ����ʵ���Ϊ ����ЩOH���� molNH3��������ͬ���� g Na+���е���������ͬ��
�����е��ӵ����ʵ���Ϊ ����ЩOH���� molNH3��������ͬ���� g Na+���е���������ͬ����1��0.2mol�� 0.2��6.02��1023�� 0.4
��2�� 1:1 ; 1:1 ; 5:3 ; 4:11
��3�� 11.2 ��14
��4��0.5mol�� 8.5g�� 4.5mol�� 5mol�� 0.5�� 11.5
��2�� 1:1 ; 1:1 ; 5:3 ; 4:11
��3�� 11.2 ��14
��4��0.5mol�� 8.5g�� 4.5mol�� 5mol�� 0.5�� 11.5
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
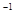 ��NaOH��Һ��ȡ��100mL��Һ�����������100mL��Һ������������ǣ� ��
��NaOH��Һ��ȡ��100mL��Һ�����������100mL��Һ������������ǣ� ��