��Ŀ����
��12�֣�ϩ������һ����ɫ�д̼�����ζ��Һ�壬����Ҫ���л��ϳ�ԭ�ϣ���ṹ��ʽΪ CH2=CH��CH2OH����ش�
��1��ϩ�����ķ���ʽΪ ��ϩ�����к��еĹ����ŵ�������____��
��2��0.3mol ϩ���������������Ʒ�Ӧ�������ɱ�״���µ����� L��
��3��д��ϩ��������ˮ��Ӧ�Ļ�ѧ����ʽ ����Ӧ����Ϊ��_____________________��
��4��ϩ������CH3CO18OH����������Ӧ�Ļ�����ʽΪ ��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽΪ ����
��1��ϩ�����ķ���ʽΪ ��ϩ�����к��еĹ����ŵ�������____��
��2��0.3mol ϩ���������������Ʒ�Ӧ�������ɱ�״���µ����� L��
��3��д��ϩ��������ˮ��Ӧ�Ļ�ѧ����ʽ ����Ӧ����Ϊ��_____________________��
��4��ϩ������CH3CO18OH����������Ӧ�Ļ�����ʽΪ ��������Ӧ���ɵIJ�����һ�������¿��Է����Ӿ۷�Ӧ�õ��߷��ӻ������ṹ��ʽΪ ����
��12�֣���1��C3H6O(1��) ̼̼˫�� �ǻ�(2��) ��2��3.36L (2��)
��3��CH2=CH��CH2OH+Br2�D��CH2BrCHBrCH2OH (2��) �ӳɷ�Ӧ��1�֣�
��4��CH3CO18O H + CH2=CH��CH2OH CH3COOCH2CH=CH2 + H218O��2�֣�
CH3COOCH2CH=CH2 + H218O��2�֣�
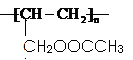 ��2�֣�
��2�֣�
��3��CH2=CH��CH2OH+Br2�D��CH2BrCHBrCH2OH (2��) �ӳɷ�Ӧ��1�֣�
��4��CH3CO18O H + CH2=CH��CH2OH
 CH3COOCH2CH=CH2 + H218O��2�֣�
CH3COOCH2CH=CH2 + H218O��2�֣�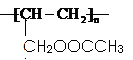 ��2�֣�
��2�֣���1�����ݽṹ��ʽ��֪��ϩ�����ķ���ʽΪC3H6O�����еĹ�������̼̼˫�����ǻ���
��2��������ֻ��1���ǻ�������0.3mol���л����������0.15mol�������ڱ�״���µ������0.15mol��22.4L/mol��3.36L��
��3������̼̼˫�������Ժ���ˮ�����ӳɷ�Ӧ��
��4����������Ӧ�У������ṩ�ǻ������ṩ��ԭ�ӣ�����������ˮ�к���18O���������ɵ����к���̼̼˫���������ܷ����Ӿ۷�Ӧ�����ɸ߷��ӻ�����ṹ��ʽΪ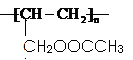 .
.
��2��������ֻ��1���ǻ�������0.3mol���л����������0.15mol�������ڱ�״���µ������0.15mol��22.4L/mol��3.36L��
��3������̼̼˫�������Ժ���ˮ�����ӳɷ�Ӧ��
��4����������Ӧ�У������ṩ�ǻ������ṩ��ԭ�ӣ�����������ˮ�к���18O���������ɵ����к���̼̼˫���������ܷ����Ӿ۷�Ӧ�����ɸ߷��ӻ�����ṹ��ʽΪ
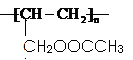 .
.
��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ




 ����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ ��
����д��A��ϡ�����KMnO4��Һ�ڼ��������·�Ӧ������Ľṹ��ʽ ��