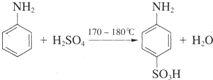
��Ŀ����
��2010?���ն�ģ�����ᣨH2C2O4����һ����Ҫ�Ļ���ԭ�ϣ���֪������0.01mol?L-1��H2C2O4��KHC2O4��K2C2O4��Һ��pH���±���ʾ��
��1����������������ʱ�����д�ʩ��ʹKHC2O4��Һ��c��K+����c��HC2O4-���ӽ�1��1����
A������������H2C2O4 B������������KHC2O4
C������������Na2C2O4 D��������Һ���¶�
��2��ȡһ������H2C2O4?2H2O��KHC2O4�Ļ�����ˮ�ܽ⣬���250mL��Һ��ȡ���ݴ���Һ��25mL�����һ����Һ���ȼ��뼸�η�̪��Һ���ٵμ�0.25mol?L-1 NaOH��Һ��20.00mLʱ����Һ����ɫ��Ϊdz��ɫ����ڶ�����Һ�еμ�����3mol?L-1 H2SO4��Һ�ữ����0.10mol?L-1 KMnO4��Һ�ζ���������KMnO4��Һ16.00mLʱ����Һ����ɫ��Ϊdz�Ϻ�ɫ����ش��������⣺
��������ӷ���ʽ��5C2O42-+2Mn04-+16H+�T10
��ԭ�������H2C2O4?2H2O��KHC2O4�����ʵ���֮��Ϊ
��3��ijʵ��С������ͼ��ʾ��װ��̽��FeC2O4?2H2O�ڸ����������������ȷֽ�IJ����36D0g FeC2O4?2H2O��ַ�Ӧ������������Ϊ13.6g��Ũ��������7.2g����ʯ������11.0g�����������ijɷֺ����ʵ���������֪FeC2O4?2H2O����Է�������Ϊ180���跴Ӧ���������屻������գ�
| H2C2O4 | KHC2O4 | K2C2O4 | |
| pH | 2.0 | 3.1 | 8.1 |
AC
AC
������ĸ����A������������H2C2O4 B������������KHC2O4
C������������Na2C2O4 D��������Һ���¶�
��2��ȡһ������H2C2O4?2H2O��KHC2O4�Ļ�����ˮ�ܽ⣬���250mL��Һ��ȡ���ݴ���Һ��25mL�����һ����Һ���ȼ��뼸�η�̪��Һ���ٵμ�0.25mol?L-1 NaOH��Һ��20.00mLʱ����Һ����ɫ��Ϊdz��ɫ����ڶ�����Һ�еμ�����3mol?L-1 H2SO4��Һ�ữ����0.10mol?L-1 KMnO4��Һ�ζ���������KMnO4��Һ16.00mLʱ����Һ����ɫ��Ϊdz�Ϻ�ɫ����ش��������⣺
��������ӷ���ʽ��5C2O42-+2Mn04-+16H+�T10
CO2
CO2
+2Mn2++8H2O����ԭ�������H2C2O4?2H2O��KHC2O4�����ʵ���֮��Ϊ
1��3
1��3
��
��3��ijʵ��С������ͼ��ʾ��װ��̽��FeC2O4?2H2O�ڸ����������������ȷֽ�IJ����36D0g FeC2O4?2H2O��ַ�Ӧ������������Ϊ13.6g��Ũ��������7.2g����ʯ������11.0g�����������ijɷֺ����ʵ���������֪FeC2O4?2H2O����Է�������Ϊ180���跴Ӧ���������屻������գ�
��������1������KHC2O4��Һ�����Կ�֪����Һ�е������ˮ�⣬ѡ�����Ƶ�����Լ���
A��H2C2O4 �ֲ����������ԣ���������HC2O4-���ӵĵ��룻
B������KHC2O4��Ȼ����Ũ���Ǵ���1��1��
C������������Na2C2O4���������� HC2O4-���ӵĵ��룻
D�����´ٽ����룻
��2�������ݵ����غ㡢����غ��ԭ���غ���ƽд����
������ԭ���غ�ͻ�ѧ����ʽ����õ���
��3�����ȼ��Ϊһ����̼���壬Ũ��������7.2gΪˮ����ʯ������11.0gΪ������̼���壮���ݷ�Ӧ���ɵ������ڹ����е������ֱ����õ���
A��H2C2O4 �ֲ����������ԣ���������HC2O4-���ӵĵ��룻
B������KHC2O4��Ȼ����Ũ���Ǵ���1��1��
C������������Na2C2O4���������� HC2O4-���ӵĵ��룻
D�����´ٽ����룻
��2�������ݵ����غ㡢����غ��ԭ���غ���ƽд����
������ԭ���غ�ͻ�ѧ����ʽ����õ���
��3�����ȼ��Ϊһ����̼���壬Ũ��������7.2gΪˮ����ʯ������11.0gΪ������̼���壮���ݷ�Ӧ���ɵ������ڹ����е������ֱ����õ���
����⣺��1��ͼ��������KHC2O4��Һ�����Կ�֪����Һ�е������ˮ�⣬ѡ������HC2O4-���ӵ�����Լ���
A��H2C2O4 �ֲ����������ԣ���������HC2O4-���ӵĵ��룬KHC2O4��Һ��c��K+����c��HC2O4-���ӽ�1��1����A���ϣ�
B������KHC2O4��Ȼ����Ũ���Ǵ���1��1����B�����ϣ�
C������������Na2C2O4���������� HC2O4-���ӵĵ��룬KHC2O4��Һ��c��K+����c��HC2O4-���ӽ�1��1����C���ϣ�
D�����´ٽ����룬KHC2O4��Һ��c��K+����c��HC2O4-������1��1����D�����ϣ�
�ʴ�Ϊ��AC��
��2��������ԭ���غ�õ�ΪCO2���ʴ�Ϊ��CO2��
��25mL�У���xmol H2C2O4?2H2O ymol KHC2O4���ݵζ��ģ����Եó���2x+y=0.25��0.02
����������ԭ�ģ����Եó���x+y=0.1��0.016��2.5��x=0.001��y=0.003mol������ԭ�������H2C2O4?2H2O��KHC2O4�����ʵ���֮��Ϊ1��3���ʴ�Ϊ��1��3��
��3������ȼ������ΪCO������Ϊm��CO��=36.0 g-13.6 g-7.2 g-11.0 g=4.2 g��
��FeC2O4?2H2OΪ0.2 mol��n��H2O��=7.2 g��18 g/mol=0.4 mol��
n��CO2��=11.0 g��44 g/mol=0.25 mol��n��CO��=4.2 g��28 g/mol=0.15 mol��
���ԣ���������������Ԫ�ؼ����ʵ���Ϊn��Fe��=0.2 mol��n��O��=0.15 mol��
���������ΪFe��FeO�Ļ�������FeΪ0.05 mol��FeOΪ0.15 mol��
�ʴ�Ϊ��FeΪ0.05 mol��FeOΪ0.15 mol��
A��H2C2O4 �ֲ����������ԣ���������HC2O4-���ӵĵ��룬KHC2O4��Һ��c��K+����c��HC2O4-���ӽ�1��1����A���ϣ�
B������KHC2O4��Ȼ����Ũ���Ǵ���1��1����B�����ϣ�
C������������Na2C2O4���������� HC2O4-���ӵĵ��룬KHC2O4��Һ��c��K+����c��HC2O4-���ӽ�1��1����C���ϣ�
D�����´ٽ����룬KHC2O4��Һ��c��K+����c��HC2O4-������1��1����D�����ϣ�
�ʴ�Ϊ��AC��
��2��������ԭ���غ�õ�ΪCO2���ʴ�Ϊ��CO2��
��25mL�У���xmol H2C2O4?2H2O ymol KHC2O4���ݵζ��ģ����Եó���2x+y=0.25��0.02
����������ԭ�ģ����Եó���x+y=0.1��0.016��2.5��x=0.001��y=0.003mol������ԭ�������H2C2O4?2H2O��KHC2O4�����ʵ���֮��Ϊ1��3���ʴ�Ϊ��1��3��
��3������ȼ������ΪCO������Ϊm��CO��=36.0 g-13.6 g-7.2 g-11.0 g=4.2 g��
��FeC2O4?2H2OΪ0.2 mol��n��H2O��=7.2 g��18 g/mol=0.4 mol��
n��CO2��=11.0 g��44 g/mol=0.25 mol��n��CO��=4.2 g��28 g/mol=0.15 mol��
���ԣ���������������Ԫ�ؼ����ʵ���Ϊn��Fe��=0.2 mol��n��O��=0.15 mol��
���������ΪFe��FeO�Ļ�������FeΪ0.05 mol��FeOΪ0.15 mol��
�ʴ�Ϊ��FeΪ0.05 mol��FeOΪ0.15 mol��
���������⿼��������ˮ���������ʵ������Դ�С�Ƚϣ�ƽ���ƶ�������жϣ�������ԭ��Ӧ���ӷ���ʽ����ƽ������ʵ����̵ļ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ