��Ŀ����
ij�л���X�ķ���ʽΪC4H8O2��X��������������ˮ��Ӧ�����������л���Y��Z��Y��ͭ���±�����ΪW��W�ܷ���������Ӧ��
(1)X��������������________(������)��
(2)д�����������X�Ľṹ��ʽ_______________________________________
________________________________________________________________________.
(3)��Y��Z������ͬ��̼ԭ������д�����з�Ӧ�Ļ�ѧ����ʽ��Y��Ũ����Ļ���ﹲ�ȷ�����ȥ��Ӧ________________________________________________________
________________________________________________________________________.
W������Cu(OH)2��Ӧ_______________________________________________��
(4)��X��ij��ͬ���칹����ʹʯ����ɫ��������________�֣�
(1)���� (2)CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH2CH2CH3
(3)CH3CH2OH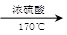 CH2===CH2����H2O CH3CHO��2Cu(OH)2
CH2===CH2����H2O CH3CHO��2Cu(OH)2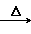 CH3COOH��Cu2O����2H2O
CH3COOH��Cu2O����2H2O
(4)2
��������
���������(1)X��������������ˮ��������л����Ϸ���ʽ��֪X�������ࡣ
(2)���ɵĴ�Y������Ϊȩ����Y�Ľṹ�к��С�CH2OHԭ���ţ�����X��CH3CH2COOCH3��CH3COOCH2CH3��HCOOCH2CH2CH3���֡�
(3)��Y��Z������ͬ��̼ԭ��������YΪCH3CH2OH��ZΪCH3COOH��WΪCH3CHO��
(4)X��ͬ���칹����ʹʯ����ɫ����ӦΪ���ᣬ��������Ľṹ�ص㣬����2�֡�
���㣺�����л���ṹ��ʽ�������š�ͬ���칹���Լ���ѧ����ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ��������֪ʶ�Ĺ�����ѵ����ͬʱ��˶�ѧ�������������ͽ��ⷽ����ָ��ѵ��������Ĺؼ������ճ��������ŵĽṹ�����ʣ��ر��ǹ�����֮����ת����ϵ������������ѧ���������������淶�Ĵ���������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�