��Ŀ����
���غ��������ŷḻ�ĺ�ˮ��Դ����ˮ����Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����CO32����HCO3�������ӡ�����������Դ�ͱ��������ǿɳ�����չ����Ҫ��֤��
 ��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ ��
��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ ��
��2��ij���������������л��������Cu2����Pb2������ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ�� ��ѡ�Na2S����NaOH����Ч�����á�
��3�������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��

����Ȼ��ˮ��pH��8���������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ�� ����дһ������
��ij�о�С��Ϊ̽����ߺ���������SO2����Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��ʾ��
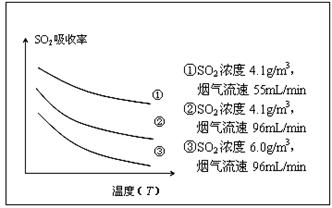
�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺 ��
����Ȼ��ˮ�����˺��������������H2SO3��HSO3���ȷ��ӻ����ӣ�ʹ���������������Ļ�ѧԭ���� ����дһ����ѧ����ʽ�����ӷ���ʽ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
����Դ����������ͷ�չ����Ҫ֧�����о���ѧ��Ӧ�����е������仯����Դ��ȱ�Ľ��������Ҫ���������塣��֪�����Ȼ�ѧ����ʽ
��2H2(g)+O2(g)��2H2O(l) H����570kJ/mol��
H����570kJ/mol��
��H2(g)+1/2O2(g)��H2O(g) H����242kJ/mol��
H����242kJ/mol��
��C(s)+1/2O2(g)��CO(g) H����110��5kJ/moL��
H����110��5kJ/moL��
��C(s)+O2(g)��CO2(g) H����393��5kJ/moL��
H����393��5kJ/moL��
��CO2(g)+2H2O(g)��2CH4(g)+2 O2(g) H��+890kJ/moL
H��+890kJ/moL
�ش���������
��1��������Ӧ���������ȷ�Ӧ���� ��
��2��H2��ȼ����Ϊ��H�� ��
��3����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳⶨ������ͨ����ӵķ�����á���֪C(s) + H2O(g)��H2(g)+ CO(g) H��akJ/moL����a�� ���÷�Ӧ����
H��akJ/moL����a�� ���÷�Ӧ���� S 0(ѡ���������������������)����֪������
S 0(ѡ���������������������)����֪������ G��
G�� H��T
H��T S����
S���� G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________��
G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________��
��4��CO��������ȼ�ϵ��Ϊ����ԭ������װ����ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2-�����ڹ������NASICON�������ƶ�������˵��������� ��
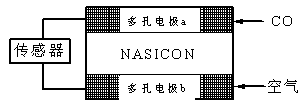
A�������ĵ缫��ӦʽΪ��CO+O2���D2e-��CO2
B������ʱ�缫b��������O2���ɵ缫a����缫b
C������ʱ�����ɵ缫aͨ������������缫b
D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ��
 ��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ ��
��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ ����2��ij���������������л��������Cu2����Pb2������ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ�� ��ѡ�Na2S����NaOH����Ч�����á�
| ���ܵ���� | Cu(OH)2 | CuS | Pb(OH)2 | PbS |
| Ksp | 4��8��10��20 | 6��3��10��36 | 1��2��10��15 | 1��0��10��28 |
��3�������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��

����Ȼ��ˮ��pH��8���������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ�� ����дһ������
��ij�о�С��Ϊ̽����ߺ���������SO2����Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��ʾ��

�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺 ��
����Ȼ��ˮ�����˺��������������H2SO3��HSO3���ȷ��ӻ����ӣ�ʹ���������������Ļ�ѧԭ���� ����дһ����ѧ����ʽ�����ӷ���ʽ����������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
����Դ����������ͷ�չ����Ҫ֧�����о���ѧ��Ӧ�����е������仯����Դ��ȱ�Ľ��������Ҫ���������塣��֪�����Ȼ�ѧ����ʽ
��2H2(g)+O2(g)��2H2O(l)
 H����570kJ/mol��
H����570kJ/mol����H2(g)+1/2O2(g)��H2O(g)
 H����242kJ/mol��
H����242kJ/mol����C(s)+1/2O2(g)��CO(g)
 H����110��5kJ/moL��
H����110��5kJ/moL����C(s)+O2(g)��CO2(g)
 H����393��5kJ/moL��
H����393��5kJ/moL����CO2(g)+2H2O(g)��2CH4(g)+2 O2(g)
 H��+890kJ/moL
H��+890kJ/moL�ش���������
��1��������Ӧ���������ȷ�Ӧ���� ��
��2��H2��ȼ����Ϊ��H�� ��
��3����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳⶨ������ͨ����ӵķ�����á���֪C(s) + H2O(g)��H2(g)+ CO(g)
 H��akJ/moL����a�� ���÷�Ӧ����
H��akJ/moL����a�� ���÷�Ӧ���� S 0(ѡ���������������������)����֪������
S 0(ѡ���������������������)����֪������ G��
G�� H��T
H��T S����
S���� G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________��
G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________����4��CO��������ȼ�ϵ��Ϊ����ԭ������װ����ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2-�����ڹ������NASICON�������ƶ�������˵��������� ��

A�������ĵ缫��ӦʽΪ��CO+O2���D2e-��CO2
B������ʱ�缫b��������O2���ɵ缫a����缫b
C������ʱ�����ɵ缫aͨ������������缫b
D����������ͨ���ĵ���Խ��β����CO�ĺ���Խ��
����15�֣���1�� MgCl2�����ڣ� Mg��Cl2��1�� ��2�� Na2S 1��
Mg��Cl2��1�� ��2�� Na2S 1��
��3����CO32����H2O HCO3����OH���� HCO3����H2O
HCO3����OH���� HCO3����H2O H2CO3��OH�� 1��
H2CO3��OH�� 1��
�ڽ��ͺ����������¶ȣ����С�������������٣��� 1��
��2H2SO3��O2��4H����2SO42������2H2SO4����2HSO3����O2��2H����2SO42����2��
�к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����������Ⱦ��1��
��1�� �� 1�� ��2����285kJ/mol 1��
��3����131.5 2�� �� 1�� ���� 1�� ��4��B 2��
 Mg��Cl2��1�� ��2�� Na2S 1��
Mg��Cl2��1�� ��2�� Na2S 1����3����CO32����H2O
 HCO3����OH���� HCO3����H2O
HCO3����OH���� HCO3����H2O H2CO3��OH�� 1��
H2CO3��OH�� 1���ڽ��ͺ����������¶ȣ����С�������������٣��� 1��
��2H2SO3��O2��4H����2SO42������2H2SO4����2HSO3����O2��2H����2SO42����2��
�к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����������Ⱦ��1��
��1�� �� 1�� ��2����285kJ/mol 1��
��3����131.5 2�� �� 1�� ���� 1�� ��4��B 2��
���������I����1��þ�ǻ��õĽ�����ұ��ʱ��Ҫͨ��������ڵ��Ȼ�þ���У���Ӧ�Ļ�ѧ����ʽΪMgCl2�����ڣ�
 Mg��Cl2����
Mg��Cl2������2�����ݱ������ݿ�֪���Ȼ�ͭ�������ܶȻ������ֱ�ԶС��������ͭ�������������ܶȻ����������Գ��������ѡ�����ơ�
��3�������ں�ˮ�к���CO32����HCO3�������ӣ����߾�ˮ��ʹ��ˮ�Լ��ԣ���Ӧ�����ӷ���ʽΪCO32����H2O
 HCO3����OH���� HCO3����H2O
HCO3����OH���� HCO3����H2O H2CO3��OH����
H2CO3��OH�����ڸ���ͼ���֪���¶�Խ�ߣ�SO2��������ԽС�����¶���ͬʱ����������ԽС��SO2��������Խ�����Ժ�������Ӧ���ǽ��ͺ����������¶ȣ����С�������������٣���
������H2SO3��HSO3���ȷ��ӻ����Ӿ����л�ԭ�ԣ��ױ�����ΪSO42������Ӧ�����ӷ���ʽΪ2H2SO3��O2��4H����2SO42������2H2SO4����2HSO3����O2��2H����2SO42�������ڷ�Ӧ����Һ��������ǿ������������ġ���ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ�����к͡�ϡ�;�����������ˮ�����ɵ��ᣨH+����������Ⱦ��
��1�����Ȼ�ѧ����ʽ�С�H��0��ʾ��Ӧ�Ƿ��ȷ�Ӧ����H��0��ʾ��Ӧ�����ȷ�Ӧ������������Ӧ���������ȷ�Ӧ���Ǣݣ�������Ƿ��ȷ�Ӧ��
��2��ȼ��������һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų�������������H2��ȼ����Ϊ��H����570kJ/mol��2����285kJ/mol��
��3����֪��H2(g)+1/2O2(g)��H2O(g) ��H����242kJ/mol����C(s)+1/2O2(g)��CO(g) ��H����110��5kJ/moL������ݸ�˹���ɿ�֪�ۣ��ڼ��õ�C(s) + H2O(g)��H2(g)+ CO(g)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H����110��5kJ/moL��242kJ/mol����131.5 kJ/mol����a����131.5�����ݷ���ʽ��֪���÷�Ӧ���������ģ����Ը÷�Ӧ���ء�S��0�����ݡ�G����H��T����S��֪������G��0ʱ���Է����С����ڸ÷�Ӧ�ġ�S��0����H��0����÷�ӦӦ���ڸ��������¿��Է����С�
��4��ԭ����и���ʧȥ���ӣ�����������Ӧ�������õ����ӣ�������ԭ��Ӧ�������ڸ�ȼ�ϵ����CO�ٸ���ͨ�룬����������ͨ�롣A��CO�ڸ���ͨ�룬���ĵ缫��ӦʽΪ��CO+O2���D2e-��CO2��A��ȷ��B������ʱ�缫b������������ԭ��������������ƶ�������O2���ɵ缫b����缫a��B����ȷ��C��ԭ����е��ӴӸ����������������Թ���ʱ�����ɵ缫aͨ������������缫b��C��ȷ��D����������ͨ���ĵ���Խ�����ĵ�COԽ�࣬����β����CO�ĺ���Խ�ߣ�D��ȷ����ѡB��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
O2(g)===H2O(l) ��H3����285.84 kJ��mol��1 N2(g)+CO2(g) ��H="Q" kJ��mol-1��
N2(g)+CO2(g) ��H="Q" kJ��mol-1��
 4CO(g) + BaS(s) ��H1 = +571��2 kJ��mol-1 ��
4CO(g) + BaS(s) ��H1 = +571��2 kJ��mol-1 ��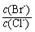 = �� ����֪��Ksp(AgBr)=5��4��10-13��Ksp(AgCl)=2��0��10-10��
= �� ����֪��Ksp(AgBr)=5��4��10-13��Ksp(AgCl)=2��0��10-10�� O2(g)=CO2(g)��2H2O(l)����H����764.5 kJ��mol��1
O2(g)=CO2(g)��2H2O(l)����H����764.5 kJ��mol��1 O2(g)=CO2(g)����H����283.0 kJ��mol��1
O2(g)=CO2(g)����H����283.0 kJ��mol��1 CH3OH(g)����H��________kJ��mol��1
CH3OH(g)����H��________kJ��mol��1
