��Ŀ����
ij����Һ�п��ܺ���Mg2+��Al3+��Fe2+��Br����![]() ��
��![]() �еļ��֣��ֽ�������ʵ�飺
�еļ��֣��ֽ�������ʵ�飺
�ٴ���Һ��ͨ��H2S������������Һ��ͨ�������Cl2�����BaCl2��Һ�����ɳ���A����Һa��
����Һa�м�����������Һ�ֲ㣬����������ʻ�ɫ������������Һ�м���KI������Һ������ɫ���֣�
����Һa�м������NaOH��Һ�����ɳ���B�����˺����Һb����b�м����������������ɳ���C��C���������ᣮ
��ȷ������Һ��һ������________��һ������________������ȷ������________��

| |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
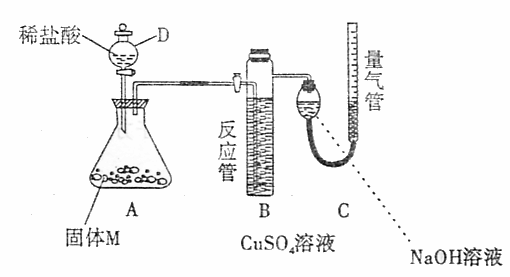
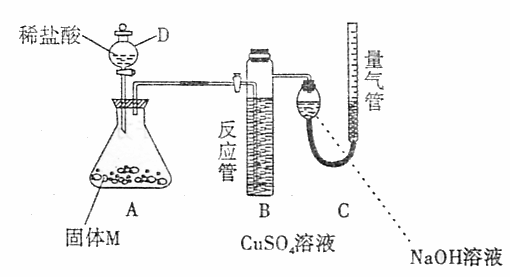
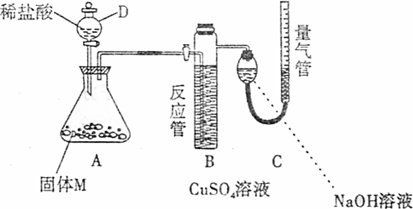
26.(A)ij������ȤС��Ϊ��̽���������ڸ��������������·�Ӧ���ù���M�ijɷ֣��������ͼװ�á���бAʹϡ����(����)�����M��ַ�Ӧ������Ӧֹͣ��Bװ�����أ�Cװ������Һ�ޱ仯����Ӧ�������������������ΪVmL��������ɱ�״����
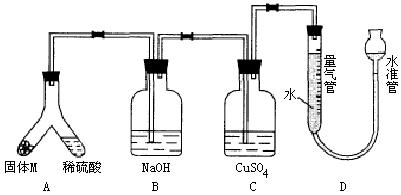
������ʵ����ʵ��֪��
(1)�ٹ���M��һ���е�������______________(�ѧʽ)
������_____________________________________________________________
������һ�����ʵ���������ȷ��Ϊ___________g(�ô���ʽ��ʾ)��
(2)Bװ�õ�������________��
д��Bװ���з�Ӧ�����ӷ���ʽ_______________________________________��
(3)Cװ�õ�������_________________________�����ʵ����û��Bװ�ã���Cװ���в�����������____________________________________________________________��
(4)ϡ�������M��Ӧ����Һ�л���������ɫ���壬�ù�����_____________��Ҫ������ù��壬��ʵ������У����ձ����Ҫ�õ��IJ���������_____________��
(5)ͨ����һ��ʵ�飬��ù���M�и��ֳɷֵ�����֮��С�ڷ�Ӧǰ���ۺ���۵�����֮�ͣ��������������ԭ�������_____________
a.M����δ��Ӧ��������
b.�ⶨ�������ʱˮ�ܵ�ˮ����������ܵ�ˮ��
c.A�����з�Ӧ���ɵ�����
d.�������Dװ��ǰδ��Ũ�������
��B��
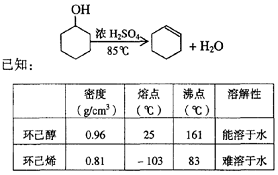
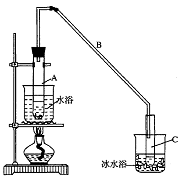
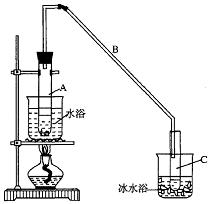
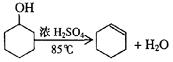
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
��֪��
| �ܶȣ�g/cm3�� | �۵㣨�棩 | �е㣨�棩 | �ܽ��� |
���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
����ϩ | 0.81 | -103 | 83 | ������ˮ |
(1)�Ʊ���Ʒ
��12.5 mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ����������
����Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����________________________________________________��
(2)�Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һ����_________(������)ϴ�ӡ�
a.KMnO4��Һ b.ϡH2SO4 c.Na2CO3��Һ
���ٽ�����ϩ����ͼװ��������ȴˮ��_________�ڽ��롣����ʱҪ������ʯ�ң�Ŀ����__________________��
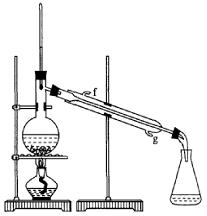
���ռ���Ʒʱ�����Ƶ��¶�Ӧ��_________���ң�ʵ���ƵõĻ���ϩ��Ʒ�����������۲��������ܵ�ԭ���ǣ� ��
a.����ʱ��70�濪ʼ�ռ���Ʒ
b.������ʵ����������
c.�Ʊ���Ʒʱ���������Ʒһ������
(3)�������ֻ���ϩ��Ʒ�ʹ�Ʒ�ķ�������������_________��
a.�����Ը��������Һ
b.�ý�����
c.�ⶨ�е�