��Ŀ����
�л���ѧ��Ӧ��Ӧ������ͬ�������ɲ�ͬ���л���Ʒ�����磺

��һ�� ����ͬϵ����±�ص��ʻ�ϣ��ڹ��������£������ϵ���ԭ�ӱ�±��ԭ��ȡ����
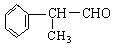
(��)��ҵ������������Ϣ��������·�ߺϳɽṹ��ʽΪ�����ʣ����������⻯����ȩһ�����ϡ�
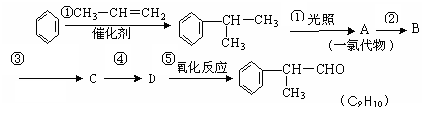 |
���������·�ߣ��ش��������⣺
��1��AΪһ�ȴ�����Ľṹ��ʽ����Ϊ_______________________________________��
��2����ҵ���������У��м������뾭���ڢۢܵõ�D��������ȡֱ��ת��ΪD�ķ���ԭ������������������������������������������������������
��3��д����Ӧ���� ��_____�������������� ____����____������������������ _____��
��4����Ӧ�ܵĻ�ѧ����ʽΪ��
_______________________________________________________________��
�⻯����ȩ������1:1�ӳɺ�IJ���C9H10O��ͬ���칹��ܶ࣬д�����ַ����������������� C9H10O��ͬ���칹��Ľṹ��ʽ��
������ˮ��Ӧ����������������Һ��Ӧ�۷�����ֻ�б���һ�ֻ�״�ṹ��������������ȡ�������ұ����ϵ�һ����������֡�����������������������
�⻯����ȩ����������Ӧ�Ļ�ѧ����ʽΪ����������������������������������������������
��1��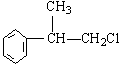 ��
��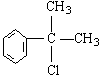 ��4�֣�
��4�֣�
��2��A�����ֽṹ��ֱ��ˮ�ⲻ�����ɴ���������ɵ��ǻ����(2��)
��3�� ��ȥ��Ӧ �ӳɷ�Ӧ ��2�֣�
��4����2�֣�
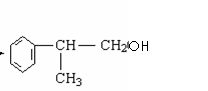
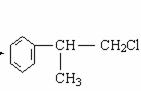
+NaOH + NaCl
![]()
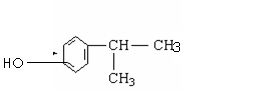 ��5����4�֣�
��5����4�֣�
��6����3�֣�
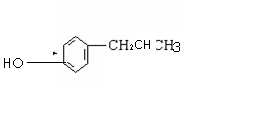 |


 �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�

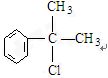


 ��ˮ�����
��ˮ����� ���ܾ�������Ӧ���õ���Ʒ����A��
���ܾ�������Ӧ���õ���Ʒ����A�� ��ˮ�����
��ˮ����� �е�-OH�������ˣ�����������-CHO��
�е�-OH�������ˣ�����������-CHO��

 �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�
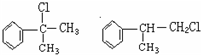
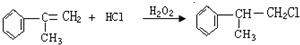

 �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�

 ��
��

 ��ˮ�����
��ˮ����� ���ܾ�������Ӧ�õ���Ʒ
���ܾ�������Ӧ�õ���Ʒ
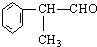 �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�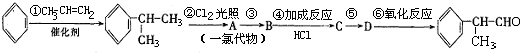
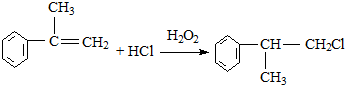
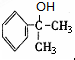 ���ɣ�
���ɣ�
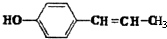

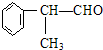 �����ʣ���������һ�����ϣ�
�����ʣ���������һ�����ϣ�
 ���Ƿ�������
���Ƿ�������