��Ŀ����
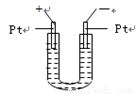
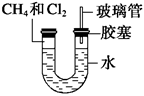
��6�֣���ͼ��ʾ��U�ιܵ���˱�ˮ�ͽ�������м���������������Ϊ1��4���Ļ�����壬�ٶ�������ˮ�е��ܽ�ȿ��Ժ��ԡ�������м���������Ļ�������װ��
�������й����ĵط����û�����建���ط�Ӧһ��ʱ�䡣
(1)�����������ķ�Ӧ���� ��Ӧ����Ӧ��õ��������л�����Ľṹ��ʽΪ ��
(2)��������Сʱ�ķ�Ӧ��U�ι��Ҷ˵�ˮ���仯��_______ ��
A�����ߡ��� B�����͡��� C�����䡡�� D����ȷ��
��6�֣�CO��CH4��Ϊ�����Ŀ�ȼ�����塣
(1)��֪��101 kPaʱ�� CH4��ȫȼ������1molҺ̬ˮ���ų�������ΪQkJ����CH4��ȫȼ�շ�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
(2)120�桢101 kPa�£�a mL��CO��CH4��ɵĻ��������bmL O2����ȫȼ�պָ���ԭ�¶Ⱥ�ѹǿ��
�����������������ǡ����ȫ��Ӧ������bmL������̼�����������һ����̼�ͼ�������ʵ���֮��Ϊ�� ��
����ȼ�պ����������С��a/4 mL����a��b��ϵ����ѧ��ʾʽ�� ��
��.(1)ȡ�� CH3Cl CH2Cl2 CHCl3 CCl4 (2)B
��. (1) CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H=��2QkJ��mol��1
(2)�� 2:1 ��b��5a/4
����:�������������ȡ����Ӧ���õ�������ȴ��������٣�ѹǿ��С��U�ι������ҽ���
CH4(g)+2O2(g)=CO2(g)+2H2O(l) ��H=��2QkJ��mol��1
2CO-----O2---------2CO2 CH4--------2O2-------CO2
2X X 2X Y 2Y Y
2X+Y=a x+2y=b x= (2a-b ) ��3 y=(2b-a ) ��3

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�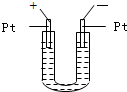 ��2006?�ϳ�һģ����ͼ��ʾ��U�ι���װ�뺬����ɫʯ���Na2SO4��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ�������
��2006?�ϳ�һģ����ͼ��ʾ��U�ι���װ�뺬����ɫʯ���Na2SO4��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ�������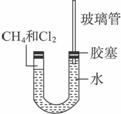
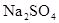 ��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ���
��
��Һ��ֱͨ���磬һ��ʱ���U�ι��ڻ��γ���ɫ���ʺ硱����������������ɫ�Ĵ����ǣ���
��