��Ŀ����
�����������仯�����֪ʶ��������и��⡣
(1)�������ʸ�����Ӧ���������ܸ�KSCN��Һ�������ɺ�ɫ��Һ����________��
A.���� B.���� C.CuSO4��Һ D.ϡ����
(2)�ѹ��������ۼ���ϡ�����У�������________��
A.����Ӧ B.���������� C.������������
(3)Ҫ��ȥFeCl2��Һ�е�����FeCl3�����еİ취��________��
A.����KSCN��Һ B.ͨ������ C.����ͭ�� D.��������
��ҵ��������Ʊ�����Ȼ������������ɼ�����ͬ�ĵط�������������·�١��ڡ����ǹ�ҵ�����������Ҫ;������·��������̵ܹ����������������;����
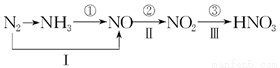
(1)д���ٲ���Ӧ�Ļ�ѧ����ʽ��____________________________________________________��
(2)���л���������NO2���ŷ��йص���________(����ĸ���)��
A���ೱ B���⻯ѧ���� C�������ն� D������ЧӦ E������
(3)����12.8 gͭ��һ������Ũ���ᷴӦ��ͭ������ʱ������������5.6 L(��״��)���������ĵ���������ʵ�����________��
 ��У����ϵ�д�
��У����ϵ�д���ҵ�����Ȼ�隣������̿��Ʊ�̼���̵���������ͼ��ʾ��
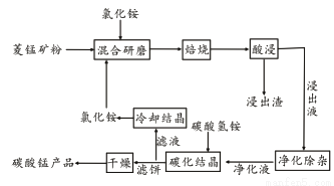
��֪�������̿����Ҫ�ɷ���MnCO3 �����к�Fe��Ca��Mg��Al��Ԫ�ء�
�ڱ��չ�������Ҫ��ӦΪ��MnCO3 +2NH4Cl  MnCl2+2NH3�� +CO2��+ H2O��
MnCl2+2NH3�� +CO2��+ H2O��
�۲��������ӳ���ʱ��Һ��pH��ֵ��
Al3- | Fe3+ | Ca2+ | Mn2+ | Mg2+ | |
��ʼ������pHֵ | 4.1 | 2.2 | 10.6 | 8.8 | 9.6 |
������ȫ��pHֵ | 4.7 | 3.2 | 13.1 | 10.1 | 11.1 |
��1��ʵ���ҡ����ա�����ʢ�Ź��������Ϊ____________________��
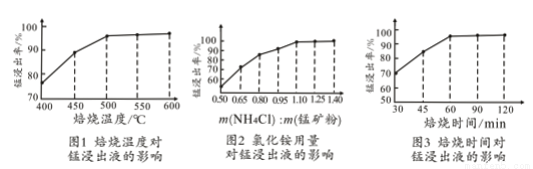
��2�����ͼ1��2��3���������չ����ж��¶ȡ�NH4Cl����[m��NH4Cl����m���̿�ۣ�]��ʱ������ѡ������Ϊ________________��_______________��______________��
��3�����̽���Һ��������ʱ���ȼ���MnO2��Fe2��ת��ΪFe3�����ٵ�����ҺpH�ķ�ΧΪ_________________����Fe3����Al3����Ϊ������ȥ��Ȼ�����NH4F��Ca2����Mg2����Ϊ�����������ȥ��
��4����̼���ᾧ�������У�����̼������Ƿ�Ӧ�����ӷ���ʽΪ________________��
��5�����������п�ѭ��ʹ�õ�������_______________��
��6��Ϊ�ⶨ��Ʒ��̼���̵ĺ������������ʵ�飨���ʲ��μӷ�Ӧ����ʵ�鲽��Ϊ����ȡ16.80g����������������ϡ������Һ�У���������Һ�м����Թ�������������ᣬ����ʹ��Ӧ��2Mn2++NO3-+4PO43-+2H+ 2[Mn(PO4)2]3-+NO2-+H2O��ֽ��С���ȥ��Һ�д��ڵ�NO3-��NO2-����l00.00mL2.00 mol��L-1��(NH4)2Fe(SO4)2��Һ�������ķ�ӦΪ��[Mn(PO4)2]3-+Fe2��=Mn2++Fe3��+2PO3-������1.00mol��L-1����K2Cr2O7��Һ�ζ�������Fe2�����ζ��յ�ʱ����10.00mL����K2Cr2O7��Һ��
2[Mn(PO4)2]3-+NO2-+H2O��ֽ��С���ȥ��Һ�д��ڵ�NO3-��NO2-����l00.00mL2.00 mol��L-1��(NH4)2Fe(SO4)2��Һ�������ķ�ӦΪ��[Mn(PO4)2]3-+Fe2��=Mn2++Fe3��+2PO3-������1.00mol��L-1����K2Cr2O7��Һ�ζ�������Fe2�����ζ��յ�ʱ����10.00mL����K2Cr2O7��Һ��
������K2Cr2O7��Һ��Fe2����Ӧ�����ӷ���ʽΪ_____________________����ԭ������Cr3������
�ڲ�Ʒ��̼���̵���������Ϊ_____________���������3λ��Ч���֣���




 Cl2��+H2��+2OH��
Cl2��+H2��+2OH��