��Ŀ����
������ˮ��Һ�е���Ϊ����ѧ��ѧ����Ҫ���ݣ���֪�������ʵĵ��볣��ֵ��25�棩��HClO��Ka=3×10-8��HCN��Ka=4.9×10-10
H2CO3��Ka1=4.3×10-7��Ka2=5.6×10-11
��1��84����Һ��ͨ��������CO2���÷�Ӧ�Ļ�ѧ����ʽΪ______��
��2��25��ʱ�������ʵ���Ũ�ȵĢ�NaClO��Һ����Na2CO3��Һ����NaCN��Һ������Һ��pH�ɴ�С��˳��Ϊ______������ţ���
��3�������ʵ���NaClO��NaHCO3�Ļ��Һ�У�����Ũ�ȵĹ�ϵΪ��[Na+]=______+[H2CO3]
��4��ʵ���ҿ�����ͼ��ʾװ���Ʊ�84����Һ����aΪ______�����Ʊ��������ܵ����ӷ�Ӧ����ʽΪ______ ClO-+H2��

���𰸡���������1����Ka��֪̼������ԣ�HClO������ǿ����ȡ����ķ�Ӧ��
��2�������ʵ���Ũ�ȵ�����Һ����Խ�������Ӧ�ε�ˮ��̶�Խ��pH��Խ��
��3�����������غ���������
��4����ͼ��֪��aΪ������bΪ���������¶������ӷŵ��������������϶����ɵ�NaOH��Һ��Ӧ����NaClO��
��5�����ݵ���غ�ʽΪ[CH3COO-]+[OH-]=[Na+]+[H+]����Һ������ʱ[OH-]=[H+]������ƽ�ⳣ��Ka=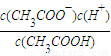 ���Դ˼��㣮
���Դ˼��㣮
����⣺��1����Ka��֪̼������ԣ�HClO����84����Һ��ͨ��������CO2�ķ�ӦΪNaClO+CO2+H2O�THClO+NaHCO3��
�ʴ�Ϊ��NaClO+CO2+H2O�THClO+NaHCO3��
��2�������ʵ���Ũ�ȵ�����Һ����Խ�������Ӧ�ε�ˮ��̶�Խ��pH��Խ����Ka��֪������HClO��HCN��HCO3-��������ʵ���Ũ�ȵĢ�NaClO��Һ����Na2CO3��Һ����NaCN��Һ������Һ��pH�ɴ�С��˳��Ϊ�ڣ��ۣ��٣��ʴ�Ϊ���ڣ��ۣ��٣�
��3�������ʵ���NaClO��NaHCO3�Ļ��Һ�У��������غ��֪��n��Na��=n��Cl��+n��C������[Na+]=[ClO-]+[HClO]+[HCO3-]+[HCO32-]+[H2CO3]��
�ʴ�Ϊ��[Na+]=[ClO-]+[HClO]+[HCO3-]+[HCO32-]��
��4����ͼ��֪��aΪ������bΪ���������¶������ӷŵ��������������϶����ɵ�NaOH��Һ��Ӧ����NaClO�������ӷ�ӦΪCl-+H2O ClO-+H2����
ClO-+H2����
�ʴ�Ϊ������Cl-+H2O ClO-+H2����
ClO-+H2����
��5�����ݵ���غ�ʽΪ[CH3COO-]+[OH-]=[Na+]+[H+]����Һ������ʱ[OH-]=[H+]����[CH3COO-]=[Na+]���ֵ���ƽ�ⳣ��Ka=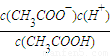 ��c��H+��=10-7mol/L��[CH3COO-]=[Na+]=0.005mol/L��c��CH3COOH��=
��c��H+��=10-7mol/L��[CH3COO-]=[Na+]=0.005mol/L��c��CH3COOH��=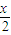 mol/L����Ka=
mol/L����Ka=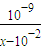 ���ʴ�Ϊ��=
���ʴ�Ϊ��=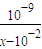 ��
��
���������⿼������ˮ���Ӧ�ü����ԭ������ȷ����ˮ����ɼ�����ǿ���Ĺ�ϵ������ƽ�ⳣ���ļ��㡢���ӵķŵ�˳��ȼ��ɽ����Ŀ�Ѷ��еȣ�
��2�������ʵ���Ũ�ȵ�����Һ����Խ�������Ӧ�ε�ˮ��̶�Խ��pH��Խ��
��3�����������غ���������
��4����ͼ��֪��aΪ������bΪ���������¶������ӷŵ��������������϶����ɵ�NaOH��Һ��Ӧ����NaClO��
��5�����ݵ���غ�ʽΪ[CH3COO-]+[OH-]=[Na+]+[H+]����Һ������ʱ[OH-]=[H+]������ƽ�ⳣ��Ka=
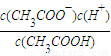 ���Դ˼��㣮
���Դ˼��㣮����⣺��1����Ka��֪̼������ԣ�HClO����84����Һ��ͨ��������CO2�ķ�ӦΪNaClO+CO2+H2O�THClO+NaHCO3��
�ʴ�Ϊ��NaClO+CO2+H2O�THClO+NaHCO3��
��2�������ʵ���Ũ�ȵ�����Һ����Խ�������Ӧ�ε�ˮ��̶�Խ��pH��Խ����Ka��֪������HClO��HCN��HCO3-��������ʵ���Ũ�ȵĢ�NaClO��Һ����Na2CO3��Һ����NaCN��Һ������Һ��pH�ɴ�С��˳��Ϊ�ڣ��ۣ��٣��ʴ�Ϊ���ڣ��ۣ��٣�
��3�������ʵ���NaClO��NaHCO3�Ļ��Һ�У��������غ��֪��n��Na��=n��Cl��+n��C������[Na+]=[ClO-]+[HClO]+[HCO3-]+[HCO32-]+[H2CO3]��
�ʴ�Ϊ��[Na+]=[ClO-]+[HClO]+[HCO3-]+[HCO32-]��
��4����ͼ��֪��aΪ������bΪ���������¶������ӷŵ��������������϶����ɵ�NaOH��Һ��Ӧ����NaClO�������ӷ�ӦΪCl-+H2O
 ClO-+H2����
ClO-+H2�����ʴ�Ϊ������Cl-+H2O
 ClO-+H2����
ClO-+H2������5�����ݵ���غ�ʽΪ[CH3COO-]+[OH-]=[Na+]+[H+]����Һ������ʱ[OH-]=[H+]����[CH3COO-]=[Na+]���ֵ���ƽ�ⳣ��Ka=
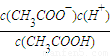 ��c��H+��=10-7mol/L��[CH3COO-]=[Na+]=0.005mol/L��c��CH3COOH��=
��c��H+��=10-7mol/L��[CH3COO-]=[Na+]=0.005mol/L��c��CH3COOH��=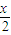 mol/L����Ka=
mol/L����Ka=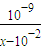 ���ʴ�Ϊ��=
���ʴ�Ϊ��=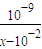 ��
�����������⿼������ˮ���Ӧ�ü����ԭ������ȷ����ˮ����ɼ�����ǿ���Ĺ�ϵ������ƽ�ⳣ���ļ��㡢���ӵķŵ�˳��ȼ��ɽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2012?��ׯ��ģ��������ˮ��Һ�е���Ϊ����ѧ��ѧ����Ҫ���ݣ���֪�������ʵĵ��볣��ֵ��25�棩��
��2012?��ׯ��ģ��������ˮ��Һ�е���Ϊ����ѧ��ѧ����Ҫ���ݣ���֪�������ʵĵ��볣��ֵ��25�棩��