��Ŀ����
ij��Һ�У�ֻ���ܺ������������еļ��֣���ÿ��ȡ100.00mL��Һ����ʵ�飺�ٵ�һ�ݼ�����������Һ�г����������ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g������˵��������ǣ� ��| ������ | K+��Mg2��Fe3+��Al3+ |
| ������ | Cl-�� �� �� |
A��C��
 ��=0.2 mol/L
��=0.2 mol/LB��C��K+��һ��Ϊ0.6mol/L
C�������ӿ��ܴ���
D��һ����
 ��
��
���𰸡��������ٵ�һ�ݼ�����������Һ�г�����������������Cl-��CO32-��SO42-�����������ɳ�����
�ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g��˵��������̼�ᱵ���������ᱵ������ԭ��Һ��һ������CO32-��SO42-���������ӹ����жϣ�Mg2��Fe3+��Al3+���ܴ��ڣ���Һ����غ㣬�������ӱ����������ӣ�����һ����K+���ӣ�����������Һ��һ������K+��CO32-��SO42-��Cl-���ܴ��ڣ�
����⣺�ٵ�һ�ݼ�����������Һ�г�����������������Cl-��CO32-��SO42-�����������ɳ�����
�ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g��˵��������̼�ᱵ���������ᱵ������ԭ��Һ��һ������CO32-��SO42-���������ӹ����жϣ�Mg2��Fe3+��Al3+���ܴ��ڣ���Һ����غ㣬�������ӱ����������ӣ�����һ����K+���ӣ�����������Һ��һ������K+��CO32-��SO42-��Cl-���ܴ��ڣ�
A��������������̼�ᱵ��������=6.27g-2.33g=3.94g�����ʵ���= =0.02mol��CO32-����Ũ��=
=0.02mol��CO32-����Ũ��= =0.2mol/L����A��ȷ��
=0.2mol/L����A��ȷ��
B�����ݼ����Ӻ�̼������Ӻ���������ӵĵ���غ���㣬������Һ��Cl-�Ĵ��ڲ�һ�������Լ����ӵ�Ũ�Ȳ�һ����0.6mol/L����B����
C�����ݷ����жϣ������ӿ��ܴ��ڣ���C��ȷ��
D���������ɳ�����Һ�����������ּ�С֤��һ������CO32-��SO42-����D��ȷ��
��ѡB��
���������⿼���˳������Ӽ����ʵ�鷽�������ӷ�Ӧ������������������ʵ�Ӧ����Һ����غ�ļ���Ӧ�ã����ӹ�����ж��ǽ���ؼ���
�ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g��˵��������̼�ᱵ���������ᱵ������ԭ��Һ��һ������CO32-��SO42-���������ӹ����жϣ�Mg2��Fe3+��Al3+���ܴ��ڣ���Һ����غ㣬�������ӱ����������ӣ�����һ����K+���ӣ�����������Һ��һ������K+��CO32-��SO42-��Cl-���ܴ��ڣ�
����⣺�ٵ�һ�ݼ�����������Һ�г�����������������Cl-��CO32-��SO42-�����������ɳ�����
�ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g��˵��������̼�ᱵ���������ᱵ������ԭ��Һ��һ������CO32-��SO42-���������ӹ����жϣ�Mg2��Fe3+��Al3+���ܴ��ڣ���Һ����غ㣬�������ӱ����������ӣ�����һ����K+���ӣ�����������Һ��һ������K+��CO32-��SO42-��Cl-���ܴ��ڣ�
A��������������̼�ᱵ��������=6.27g-2.33g=3.94g�����ʵ���=
 =0.02mol��CO32-����Ũ��=
=0.02mol��CO32-����Ũ��= =0.2mol/L����A��ȷ��
=0.2mol/L����A��ȷ��B�����ݼ����Ӻ�̼������Ӻ���������ӵĵ���غ���㣬������Һ��Cl-�Ĵ��ڲ�һ�������Լ����ӵ�Ũ�Ȳ�һ����0.6mol/L����B����
C�����ݷ����жϣ������ӿ��ܴ��ڣ���C��ȷ��
D���������ɳ�����Һ�����������ּ�С֤��һ������CO32-��SO42-����D��ȷ��
��ѡB��
���������⿼���˳������Ӽ����ʵ�鷽�������ӷ�Ӧ������������������ʵ�Ӧ����Һ����غ�ļ���Ӧ�ã����ӹ�����ж��ǽ���ؼ���

��ϰ��ϵ�д�
�����Ŀ
��2013?����ģ�⣩ij��Һ�У�ֻ���ܺ������������еļ��֣���ÿ��ȡ100.00mL��Һ����ʵ�飺�ٵ�һ�ݼ�����������Һ�г����������ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g������˵��������ǣ�������
|
ij��Һ�У�ֻ���ܺ������������еļ��֣�
| ������ | K+��Mg2��Fe3+��Al3+ |
| ������ |  �� �� �� ��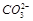 |
A��C��
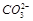 ��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L
��="0.2" mol/L B��C(K+)һ��Ϊ0.6mol/L C�������ӿ��ܴ��� D��һ����
 ��
��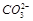
ij��Һ�У�ֻ���ܺ������������еļ��֣�
|
������ |
K+��Mg2��Fe3+��Al3+ |
|
������ |
|
��ÿ��ȡ100.00mL��Һ����ʵ�飺�ٵ�һ�ݼ�����������Һ�г����������ڵڶ��ݼ��������Ȼ�����Һ��ø������6.27g����������������ϴ�ӣ������ʣ��2.33g������˵��������ǣ� ��
A��C�� ��=0.2 mol/L B��C(K+)һ��Ϊ0.6mol/L
��=0.2 mol/L B��C(K+)һ��Ϊ0.6mol/L
C�������ӿ��ܴ��� D��һ����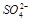 ��
��
 ��
��