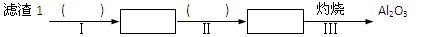��Ŀ����
(12��)˫��ˮ����������ǹ�ҵ�ϳ��õ�������������������ҵ���Բ�Ϊ������Ǧ��ʯīΪ���������NH4HSO4��Һ�ù�����李�(NH4)2S2O8����Һ���乤������Ϊ��
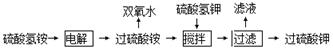
��1�����������Һ����ˮ�������ڼ�ѹ������ˮ�⡢����Ũ�����룬����ù��������ˮ��Һ��ʣ����Һ��ѭ��ʹ�á�
��д�����NH4HSO4��Һ�Ļ�ѧ����ʽ
��д��ˮ������(NH4)2S2O8��Һ��ˮ�ⷽ��ʽ ��
�۲��۸��Է�����������ò�����Ǧ��ԭ�� ��
���Է���ˮ������ʹ�ü�ѹˮ�⡢�����ԭ�� ��
��2���ڵ���Ĺ��������Һ�м���������أ�����������ع��壬������ؾ���ǿ�����ԣ�������ԭΪ����أ�80�����������ֽ⡣
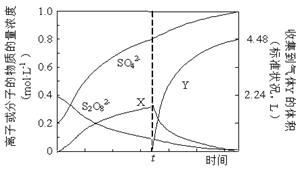
�ٽ�0.40 mol���������0.20 mol�������Ƴ�1 L��Һ����80 �������¼��Ȳ���tʱ������Һ�еμ�������FeCl3��Һ���ⶨ��Һ�и��ɷֵ�Ũ������ͼ��ʾ��H+Ũ��δ��������ͼ������X�Ļ�ѧʽΪ ��
����֪�����̣�MnSO4��������أ�K2S2O7����������Һ�������Ӵ��¿ɷ�����Ӧ���õ��Ϻ�ɫ��Һ���˷�Ӧ�����ӷ�Ӧ����ʽ .

��1�����������Һ����ˮ�������ڼ�ѹ������ˮ�⡢����Ũ�����룬����ù��������ˮ��Һ��ʣ����Һ��ѭ��ʹ�á�
��д�����NH4HSO4��Һ�Ļ�ѧ����ʽ
��д��ˮ������(NH4)2S2O8��Һ��ˮ�ⷽ��ʽ ��
�۲��۸��Է�����������ò�����Ǧ��ԭ�� ��
���Է���ˮ������ʹ�ü�ѹˮ�⡢�����ԭ�� ��
��2���ڵ���Ĺ��������Һ�м���������أ�����������ع��壬������ؾ���ǿ�����ԣ�������ԭΪ����أ�80�����������ֽ⡣
�ٽ�0.40 mol���������0.20 mol�������Ƴ�1 L��Һ����80 �������¼��Ȳ���tʱ������Һ�еμ�������FeCl3��Һ���ⶨ��Һ�и��ɷֵ�Ũ������ͼ��ʾ��H+Ũ��δ��������ͼ������X�Ļ�ѧʽΪ ��

����֪�����̣�MnSO4��������أ�K2S2O7����������Һ�������Ӵ��¿ɷ�����Ӧ���õ��Ϻ�ɫ��Һ���˷�Ӧ�����ӷ�Ӧ����ʽ .
��1����2NH4HSO4 (NH4)2S2O8��H2��2�֣�
(NH4)2S2O8��H2��2�֣�
��(NH4)2S2O8��2H2O = 2NH4HSO4+H2O2��2��
��Ǧ��������ʧ�������������ӽ�����Һ�������������ɵ�S2O82-������Ǧ��2�֣�
�ܼ�ѹˮ�⡢������Ϊ�˼���˫��ˮ�ķֽ���ʧ����2�֣�
��2����H2O2 ��2�֣�
��2Mn2����5S2O82����8H2O 2MnO4����10SO42����16H����2�֣�
2MnO4����10SO42����16H����2�֣�
 (NH4)2S2O8��H2��2�֣�
(NH4)2S2O8��H2��2�֣���(NH4)2S2O8��2H2O = 2NH4HSO4+H2O2��2��
��Ǧ��������ʧ�������������ӽ�����Һ�������������ɵ�S2O82-������Ǧ��2�֣�
�ܼ�ѹˮ�⡢������Ϊ�˼���˫��ˮ�ķֽ���ʧ����2�֣�
��2����H2O2 ��2�֣�
��2Mn2����5S2O82����8H2O
 2MnO4����10SO42����16H����2�֣�
2MnO4����10SO42����16H����2�֣������������1������ˮ��ķ�Ӧʽ���ɸ�������������Ӧ�����������д���ɵâ�2NH4HSO4
 (NH4)2S2O8��H2����(NH4)2S2O8��2H2O = 2NH4HSO4+H2O2����ʹ�ò���ԭ���ǣ�Ǧ��������ʧ�������������ӽ�����Һ�������������ɵ�S2O82-������Ǧ���ܼ�ѹˮ�⡢������Ϊ�˼���˫��ˮ�ķֽ���ʧ����2���ٸտ�ʼ��S2O82��������Ӧ������H2O2 ����H2O2 �ֽ⣬��������FeCl3��Һ�����ǵ����˴������ӿ���H2O2 �ķֽ⣬����tʱ����Ѹ�ٽ��͡�������X����H2O2 ���ڵõ��Ϻ�ɫ��Һ�����и���������ɣ��ʷ�Ӧ����ʽΪ2Mn2����5S2O82����8H2O
(NH4)2S2O8��H2����(NH4)2S2O8��2H2O = 2NH4HSO4+H2O2����ʹ�ò���ԭ���ǣ�Ǧ��������ʧ�������������ӽ�����Һ�������������ɵ�S2O82-������Ǧ���ܼ�ѹˮ�⡢������Ϊ�˼���˫��ˮ�ķֽ���ʧ����2���ٸտ�ʼ��S2O82��������Ӧ������H2O2 ����H2O2 �ֽ⣬��������FeCl3��Һ�����ǵ����˴������ӿ���H2O2 �ķֽ⣬����tʱ����Ѹ�ٽ��͡�������X����H2O2 ���ڵõ��Ϻ�ɫ��Һ�����и���������ɣ��ʷ�Ӧ����ʽΪ2Mn2����5S2O82����8H2O  2MnO4����10SO42����16H��
2MnO4����10SO42����16H���������������ڳ��湤�������⣬��Ҫ���û�ѧ��Ӧԭ�����������ս��з����������漰�Ļ�ѧԭ�����dz�����ѧ���ʵ����ʣ���������С����漰��ͬ��֪ʶ�������Ҫ���õ����ʵ����⻯ѧ���ʣ�ѧ��Ӧ�����ⲿ��֪ʶ�������գ�����ͼ���⣬���ǽ�ͼ����Ϣת��Ϊ��ѧ��������Ϣ�����⡣

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ
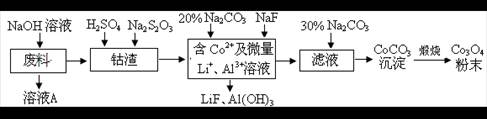
 LiF�����÷�Ӧ��ƽ�ⳣ������ʽΪK= ��
LiF�����÷�Ӧ��ƽ�ⳣ������ʽΪK= ��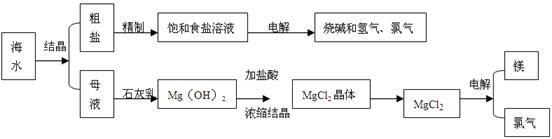
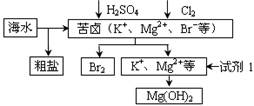
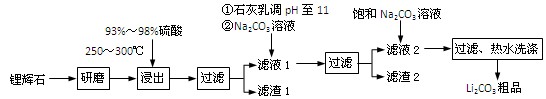
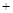 H2SO4��Ũ��
H2SO4��Ũ��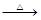 Li2SO4
Li2SO4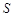 (Li2CO3)/g
(Li2CO3)/g