��Ŀ����
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⣺
(1)��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��CH4(g)��CO2(g)=2CO(g)��2H2(g)����H����260 kJ��mol��1
��֪��2CO(g)��O2(g)=2CO2(g) ��H����566 kJ��mol��1
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ_______________________________
(2)����ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ��(�������ҺΪKOH��Һ)��ͨ��װ�â�ʵ�������϶�ͭ��

��a��Ӧͨ��________(�CH4����O2��)��b���缫�Ϸ����ĵ缫��Ӧʽ��______________________________
�ڵ�ƽ�����װ�â�����Һ��pH________(��д�������С�����䡱����ͬ)��װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________(����ˮ��)��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L(��״����)��
(1)��¯ұ�������У������ڴ���Ӧ���в���ˮú��(CO��H2)��ԭ���������йط�ӦΪ��CH4(g)��CO2(g)=2CO(g)��2H2(g)����H����260 kJ��mol��1
��֪��2CO(g)��O2(g)=2CO2(g) ��H����566 kJ��mol��1
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ_______________________________
(2)����ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ��(�������ҺΪKOH��Һ)��ͨ��װ�â�ʵ�������϶�ͭ��

��a��Ӧͨ��________(�CH4����O2��)��b���缫�Ϸ����ĵ缫��Ӧʽ��______________________________
�ڵ�ƽ�����װ�â�����Һ��pH________(��д�������С�����䡱����ͬ)��װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________(����ˮ��)��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L(��״����)��
(1)2CH4(g)��O2(g)=2CO(g)��4H2(g)
��H����46 kJ��mol��1
(2)��CH4��O2��2H2O��4e��=4OH�����ڱ�С
���䡡��CO ����1.12
����1.12
��H����46 kJ��mol��1
(2)��CH4��O2��2H2O��4e��=4OH�����ڱ�С
���䡡��CO
 ����1.12
����1.12(1)���ݸ�˹���ɣ�����һ���Ȼ�ѧ����ʽ����2����ڶ�����ӵ�2CH4(g)��O2(g)=2CO(g)��4H2(g)����H����46 kJ��mol��1��
(2)����Fe���϶�Cu���� Cu������������Ӧ����������b�缫��������a�缫��������CH4��a��ͨ�룬O2��b��ͨ�룬����KOH���������Һ����b����ӦʽΪO2��2H2O��4e��=4OH������ƹ����е������Һ������缫��Ӧ��������Ũ�Ⱦ����䣻CH4ȼ�ϵ���еĻ�ѧ����ʽΪCH4��2O2��2KOH=K2CO3��3H2O���ɵ���Һ�д���CO �����ɵ����غ��CH4��4Cu����12.8 g Cu�����ʵ���Ϊ0.2 mol������CH4Ϊ
�����ɵ����غ��CH4��4Cu����12.8 g Cu�����ʵ���Ϊ0.2 mol������CH4Ϊ ��0.05 mol���ڱ�״���µ����Ϊ0.05 mol��22.4 L��mol��1��1.12 L��
��0.05 mol���ڱ�״���µ����Ϊ0.05 mol��22.4 L��mol��1��1.12 L��
(2)����Fe���϶�Cu���� Cu������������Ӧ����������b�缫��������a�缫��������CH4��a��ͨ�룬O2��b��ͨ�룬����KOH���������Һ����b����ӦʽΪO2��2H2O��4e��=4OH������ƹ����е������Һ������缫��Ӧ��������Ũ�Ⱦ����䣻CH4ȼ�ϵ���еĻ�ѧ����ʽΪCH4��2O2��2KOH=K2CO3��3H2O���ɵ���Һ�д���CO
 �����ɵ����غ��CH4��4Cu����12.8 g Cu�����ʵ���Ϊ0.2 mol������CH4Ϊ
�����ɵ����غ��CH4��4Cu����12.8 g Cu�����ʵ���Ϊ0.2 mol������CH4Ϊ ��0.05 mol���ڱ�״���µ����Ϊ0.05 mol��22.4 L��mol��1��1.12 L��
��0.05 mol���ڱ�״���µ����Ϊ0.05 mol��22.4 L��mol��1��1.12 L��
��ϰ��ϵ�д�
 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
�����Ŀ
 HOI(aq) ��H2
HOI(aq) ��H2



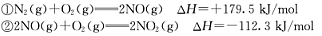


 2CO2(g)+N2(g)
2CO2(g)+N2(g)




 CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� ��
CH3OH(g)����ƽ��ʱ�����CO��H2��CH3OH�ֱ�Ϊ1 mol��1 mol��1 mol�����������Ϊ3L�����������м���ͨ��3 mol CO����ʱv������ v���棩��ѡ���>������<������=�������жϵ����� �� ������Ӧ�Ļ�ѧ��ӦΪ��__ ___
������Ӧ�Ļ�ѧ��ӦΪ��__ ___ CH3OCH3(g��+ 3H2O(g)
CH3OCH3(g��+ 3H2O(g) 2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��
2CO2(g)+N2(g)�����ܱ������з����÷�Ӧʱ,c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯����,��ͼ��ʾ��


 N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol
N2(g)+CO2(g)+2H2O(g) ��H1="-867" kJ/mol N2O4(g) ��H2="-56.9" kJ/mol
N2O4(g) ��H2="-56.9" kJ/mol