��Ŀ����
 Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ�ı�ǩ��ijѧ������ʵ����������500mL��������ע��Һ��
Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%�������ǣ�C6H12O6��ע��Һ�ı�ǩ��ijѧ������ʵ����������500mL��������ע��Һ��ʵ����Ʒ�������ǹ��塢����ˮ���ձ�������ƿ��500mL����ҩ�ס���ͷ�ιܡ���Ͳ��
��1����ȱ�ٵ�������
��2�����ж�����ƿ��ʹ�õ������в���ȷ����
A������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
B��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
C������ƿ������ˮϴ��������5%������ע��Һϴ
D��������Һʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2cm�������ý�ͷ�ιܼ�����ˮ���̶���
��3��ʵ������ȡ�ù��������Ϊ
��4�����Ƹ�������ע��Һ�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ���
A��û�н�ϴ��Һת�Ƶ�����ƿ B������ʱ���ӿ̶���
C������ƿϴ����δ���� D������ʱҺ�泬���˿̶��ߣ�
��2��A������ƿ�ϱ����ݻ����¶ȺͿ̶��ߣ�
B�����������ߵ������·���ҡ�ȣ�ʹ��ǰҪ�������ƿ�Ƿ�©ˮ��
C���ñ�������ע��Һ��ϴ������ƿ�ڱ�մ�����������ǣ�
D�������ܽ��ϡ�ͻ�������ЧӦ������Һ�����Ӱ�죬����ʹ����ƿ���Ȳ����������ѵ�Σ�գ�
��3������m=��V������Һ��������������m�����ʣ�=m����Һ�����أ����ʣ����������ǵ�������
����n=
| m |
| M |
| n |
| V |
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����c=
| n |
| V |
������Ҫ������Ϊ��������ƽ���ձ���ҩ�ס���������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��������ƽ����������
��2��A������ƿ�ϱ����ݻ����¶ȺͿ̶��ߣ���A����
B�����������ߵ������·���ҡ�ȣ�ʹ��ǰҪ�������ƿ�Ƿ�©ˮ����B��ȷ��
C���ñ�������ע��Һ��ϴ������ƿ�ڱ�մ�����������ǣ�������������Һ��Ũ��ƫ�ߣ���C����
D�������ܽ��ϡ�ͻ�������ЧӦ������Һ�����Ӱ�죬����ʹ����ƿ���Ȳ����������ѵ�Σ�գ�����ƿ����ֱ�������ܽ���壬��D����
�ʴ�Ϊ��ACD��
��3�������ǣ�C6H12O6��ע��Һ���ܶ�Ϊ1.08g?mL-1��500mL��Һ�������ǵ���������5%���������ǵ�����Ϊ��500mL��1.08g/cm3��5%=27.0g�������ǵ����ʵ���Ϊ
| 27g |
| 180g/mol |
| 0.15mol |
| 0.5L |
��4��A��û�н�ϴ��Һת�Ƶ�����ƿ����������ƿ�����ʵ����ʵ�����С��������Һ��Ũ��ƫ�ͣ���A�����ϣ�
����ƿ��ԭ����������ˮ����A����
B������ʱ���ӿ̶��ߣ�Һ���ٿ̶����·���������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ���B���ϣ�
C���������ˮ���ݣ�����ƿϴ����δ�������������ʵ�������Ӱ�죬��������ҺŨ����Ӱ�죬��C�����ϣ�
D������ʱҺ�泬���˿̶��ߣ���Һ�����ƫ��������Һ��Ũ��ƫ�ͣ���D�����ϣ�
�ʴ�Ϊ��B��
| n |
| V |

 �żӾ���ϵ�д�
�żӾ���ϵ�д�(10��)Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ�ı�ǩ��ijѧ������ʵ����������500 mL��������ע��Һ��
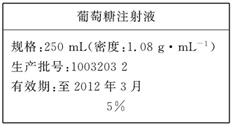
| ������ע��Һ |
| ���250mL(�ܶȣ�1.08g·mL-1) �������ţ�1003203 2 ��Ч�ڣ���2013��10�� 5% |
(1)��ȱ�ٵ������� ��
(2)���ж�����ƿ��ʹ�õ������в���ȷ���� ��
A������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
B��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
C������ƿ������ˮϴ��������5%������ע��Һϴ
D��������Һʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶���
(3)ʵ������ȡ�ù��������Ϊ g����������ע��Һ�����ʵ���Ũ�� mol/L.��
(4)���Ƹ�������ע��Һ�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ��� ��
A��û�н�ϴ��Һת�Ƶ�����ƿ B������ʱ���ӿ̶���
C������ƿϴ����δ���� D������ʱҺ�泬���˿̶���
(10��)Ϊά������ѪҺ�е�Ѫ�Ǻ������ڸ�������Һʱ��ͨ����������ע��Һ����ͼ��ҽԺ��������Һʱʹ�õ�һƿ��������Ϊ5%��������(C6H12O6)ע��Һ�ı�ǩ��ijѧ������ʵ����������500 mL��������ע��Һ��
|
������ע��Һ |
|
���250mL(�ܶȣ�1.08g��mL-1) �������ţ�1003203 2 ��Ч�ڣ���2013��10�� 5% |
ʵ����Ʒ�������ǹ��塢����ˮ���ձ�������ƿ(500 mL)��ҩ�ס���ͷ�ιܡ���Ͳ��
(1)��ȱ�ٵ������� ��
(2)���ж�����ƿ��ʹ�õ������в���ȷ���� ��
A������ƿ�ϱ����ݻ����¶Ⱥ�Ũ��
B��ʹ��ǰҪ�������ƿ�Ƿ�©ˮ
C������ƿ������ˮϴ��������5%������ע��Һϴ
D��������Һʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶���
(3)ʵ������ȡ�ù��������Ϊ g����������ע��Һ�����ʵ���Ũ�� mol/L.��
(4)���Ƹ�������ע��Һ�����в����ᵼ��������Һ�����ʵ���Ũ��ƫ�ߵ��� ��
A��û�н�ϴ��Һת�Ƶ�����ƿ B������ʱ���ӿ̶���
C������ƿϴ����δ���� D������ʱҺ�泬���˿̶���
 ʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶���
ʱ���ѳƺõ������Ǿ���С�ĵ�������ƿ�У���������ˮ���ӽ��̶���1��2 cm�������ý�ͷ�ιܼ�����ˮ���̶��� ��δ���� D������ʱҺ�泬���˿̶���
��δ���� D������ʱҺ�泬���˿̶���