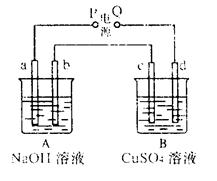
��Ŀ����
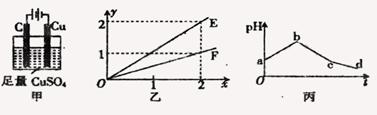
��6�֣������µ��200mLһ��Ũ�ȵ�NaCl��CuSO4�����Һ��������������������������ʱ��仯�Ĺ�ϵ����ͼ��ʾ(��������ѻ���ɱ�״���µ����)������ͼ����Ϣ�ش��������⡣
��ͨ�������Ʋ⣺
��ԭ�����ҺNaCl��CuSO4�����ʵ���Ũ�ȡ�
��t2ʱ������Һ�����������ʵ���Ũ��
��ʵ���з��֣��������������������������ȣ�����С�ڶ�Ӧʱ��ε�����ֵ���Լ�Ҫ���������ԭ��

��ͨ�������Ʋ⣺
��ԭ�����ҺNaCl��CuSO4�����ʵ���Ũ�ȡ�
��t2ʱ������Һ�����������ʵ���Ũ��
��ʵ���з��֣��������������������������ȣ�����С�ڶ�Ӧʱ��ε�����ֵ���Լ�Ҫ���������ԭ��
��1����c(NaCl)=0.1mol/L c(CuSO4)��0.1mol/L
��0.1mol/L
��2��������������������������������ˮ�е��ܽ�����Դ��������������������������������������ȣ�����С�ڶ�Ӧʱ��ε�����ֵ��
��0.1mol/L
��2��������������������������������ˮ�е��ܽ�����Դ��������������������������������������ȣ�����С�ڶ�Ӧʱ��ε�����ֵ��
������ص��йؼ��㡣����Ĺؼ��Ƕ�ͼ��Ľ�����տ�ʼʱ����Cu2+�õ��ӣ�������ų���Cu2+��Ӧ����Һ�е�H+�ŵ磬����H2������������Һ�е�Cl-�ŵ磬��Ӧ�����Һ�е�OH-�ŵ磬����ʱץס�����غ㡣
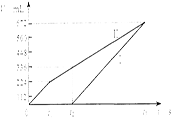
��1���ٸ���ͼ���֪������������224ml�����ʵ�����0.01mol
�����Ȼ��Ƶ�Ũ����c(NaCl)��0.01mol��2��0.2L��0.1mol/L
����ʼ��������ʱ��ͭ���ӷŵ���ϣ���ʱ����������336ml��224ml��112ml
���Թ�ת�Ƶ�����0.01mol��2��0.112L��22.4L/mol��4��0.04mol
���ݵ�ʧ��ʧ�غ��֪������ͭ�����ʵ�����0.04mol��2��0.02mol
��c(CuSO4)=0.02mol��0.2L��0.1mol/L
��t1��t2ʱ�Ų��������ӣ����ʵ�����0.112L��22.4L/mol��4��0.02mol
����������Ũ����0.02mol��0.2L��0.1mol/L
���pH��1
��1���ٸ���ͼ���֪������������224ml�����ʵ�����0.01mol
�����Ȼ��Ƶ�Ũ����c(NaCl)��0.01mol��2��0.2L��0.1mol/L
����ʼ��������ʱ��ͭ���ӷŵ���ϣ���ʱ����������336ml��224ml��112ml
���Թ�ת�Ƶ�����0.01mol��2��0.112L��22.4L/mol��4��0.04mol
���ݵ�ʧ��ʧ�غ��֪������ͭ�����ʵ�����0.04mol��2��0.02mol
��c(CuSO4)=0.02mol��0.2L��0.1mol/L
��t1��t2ʱ�Ų��������ӣ����ʵ�����0.112L��22.4L/mol��4��0.02mol
����������Ũ����0.02mol��0.2L��0.1mol/L
���pH��1

��ϰ��ϵ�д�
�����Ŀ