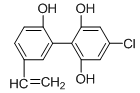
��Ŀ����
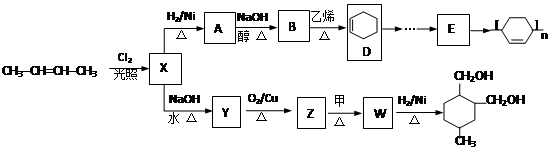
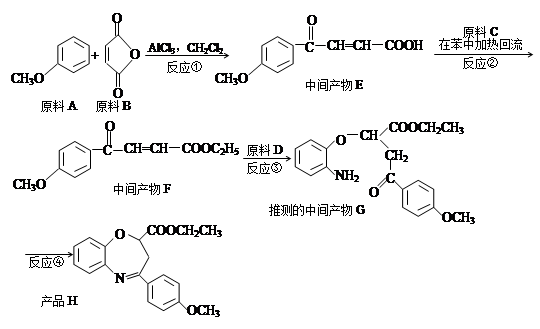
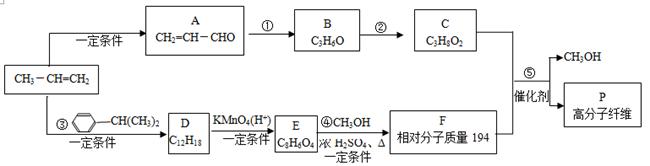
�л���A��B��C��D��E��F��G�����ϵ����ͼ��ʾ��
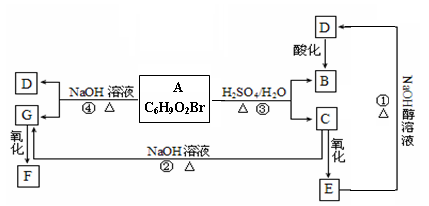
��1������A��±Ԫ�ص�ʵ�鷽���� ��
��2��B�Ľṹ��ʽΪ ���ٵĻ�ѧ��Ӧ������ ��
��3��G��ʵ�����п�ͨ��������Ӧ������F�����е�һ����Ӧ�������� ����Ӧ�õ��IJ�������У���д�ṹ��ʽ�� ��
��4��F��һ�ֶ�Ԫ�ᣬ����һ�������¿���G��Ӧ���ɸ߷��ӻ�����ø߷��ӵĽṹ��ʽΪ ��
д����Ӧ�ܵĻ�ѧ��Ӧ����ʽ ��

��1������A��±Ԫ�ص�ʵ�鷽���� ��
��2��B�Ľṹ��ʽΪ ���ٵĻ�ѧ��Ӧ������ ��
��3��G��ʵ�����п�ͨ��������Ӧ������F�����е�һ����Ӧ�������� ����Ӧ�õ��IJ�������У���д�ṹ��ʽ�� ��
��4��F��һ�ֶ�Ԫ�ᣬ����һ�������¿���G��Ӧ���ɸ߷��ӻ�����ø߷��ӵĽṹ��ʽΪ ��
д����Ӧ�ܵĻ�ѧ��Ӧ����ʽ ��
��1��ȡ����A���Թܣ���������������Һ����һ��ʱ�䣬��ȴ������ϡ��������Һ��������������������Һ������е���ɫ�������֣���A�к���Ԫ�ء���2�֣�
��2��H2C=CH-COOH��1�֣��� ��ȥ��������Ӧ��1�֣�
��3��Cu��Ag�����������ȣ�1�֣��� HO-CH2-CH2-CHO��OHC-CH2-CHO��2�֣�
��4�� ��1�֣�
��1�֣�
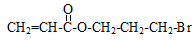 +2NaOH
+2NaOH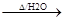 CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr��2�֣���Ӧ�������ȷ1�֣���ƽ+����1�֣�
CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr��2�֣���Ӧ�������ȷ1�֣���ƽ+����1�֣�
��2��H2C=CH-COOH��1�֣��� ��ȥ��������Ӧ��1�֣�
��3��Cu��Ag�����������ȣ�1�֣��� HO-CH2-CH2-CHO��OHC-CH2-CHO��2�֣�
��4��
 ��1�֣�
��1�֣�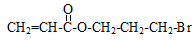 +2NaOH
+2NaOH CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr��2�֣���Ӧ�������ȷ1�֣���ƽ+����1�֣�
CH2=CHCOONa+HO-CH2-CH2-CH2-OH+NaBr��2�֣���Ӧ�������ȷ1�֣���ƽ+����1�֣������������1��±������±�����ӵļ��鷽���ǣ�ȡ����A���Թܣ���������������Һ����һ��ʱ�䣬��ȴ�����ϡ��������Һ�����ԣ��μ���������Һ������е���ɫ�������֣���A�к���Ԫ�ء�����A��������������������������ʣ���֪�Ƿ�����ˮ�ⷴӦ��D��B���ữ��˵��B���ᣬ��C�Ǵ�����̼ԭ������̼�ĹǼ���ȫ��ͬ������ת����ϵ��֪BΪH2C=CH-COOH��F��һ�ֶ�Ԫ�ᣬͨ��ת����ϵ�ɵó�CΪBrH2C-CH2-CH2OH��EΪBrH2C-CH2-CHO��DΪH2C=CH-COONa��GΪHOH2C-CH2-CH2OH,FΪHOOC-CH2-COOH��
��2��B�Ľṹ��ʽΪH2C=CH-COOH���ٵĻ�ѧ��Ӧ��������ȥ��������Ӧ��G��ʵ�����п�ͨ��������Ӧ������F�����е�һ����Ӧ��������Cu��Ag�����������ȣ���Ӧ�õ��IJ����в���������ȫ���������ʲ��������HO-CH2-CH2-CHO��OHC-CH2-CHO��
��4��F��һ�ֶ�Ԫ�ᣬ����һ�������¿���G��Ӧ���ɸ߷��ӻ�����൱�ڷ�����������Ӧ�����˸߾��д����Ӧ��Ϊ����±������ˮ�ⷴӦ�������������ŷ����˱仯�����㣺

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ
 ��
��

 ��5��F��G�ķ�Ӧ������ ��
��5��F��G�ķ�Ӧ������ ��
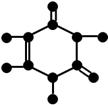
 �ں˴Ź�������ͼ�У����������Ŀ��ӳ���л���������ԭ�ӻ�ѧ���������ࣻ��ͬ�������ǿ�ȱȣ���������ĸ߶ȱȣ���ӳ�˲�ͬ��ѧ������ԭ�ӵ���Ŀ�ȡ�
�ں˴Ź�������ͼ�У����������Ŀ��ӳ���л���������ԭ�ӻ�ѧ���������ࣻ��ͬ�������ǿ�ȱȣ���������ĸ߶ȱȣ���ӳ�˲�ͬ��ѧ������ԭ�ӵ���Ŀ�ȡ�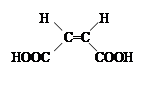 ���������������Ծ����б仯�ֱ�õ�ƻ���ᣨ
���������������Ծ����б仯�ֱ�õ�ƻ���ᣨ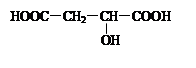 ���;ۺ���Q��
���;ۺ���Q��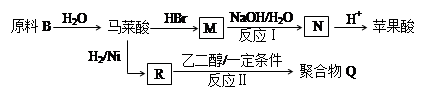

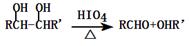
 RCO18OR"��R'OH
RCO18OR"��R'OH