��Ŀ����
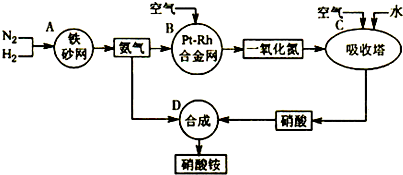
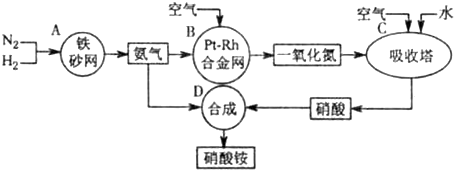
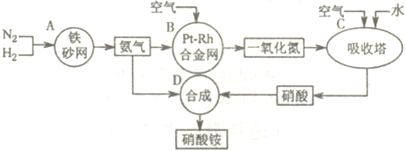
��ͼ�ǹ�ҵ��������淋�����ʾ��ͼ��
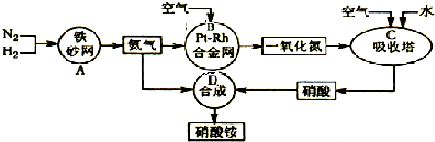
��1��������C��ͨ�����������Ŀ����______��A��B��C��D�ĸ������еķ�Ӧ������������ԭ��Ӧ����______������ĸ����
��2����֪��4NH3��g��+3O2��g���T2N2��g��+6H2O��g������H=-1266.8kJ/mol��
N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol
�ݴ�д�������´��������Ȼ�ѧ����ʽ��______��
����������������ת����5mol���ӣ���Ӧ�������仯Ϊ______kJ��
��3���ڻ����о��У�����Ҫ�жϷ�Ӧ�ܷ��Է����У���ij��Ӧ�ġ�H��0����÷�Ӧ�Ƿ�һ�����Է����У�______���һ������һ������
��4����֪��N2��g��+3H2��g��?2NH3��g������H=-92kJ/mol��Ϊ���������ת���ʣ��˲�ȡ�Ĵ�ʩ��______������ĸ����
A���ʵ������¶ȡ�
B��ʹ�ø���Ч�Ĵ�����
C������ѹǿ��
D��ѭ�����úͲ��ϲ��䵪������
E����ʱ���������
��5����һ���¶Ⱥ�ѹǿ�£���H2��N2��3��1����������ܱ������л�ϣ�����Ӧ�ﵽƽ��ʱ�����ƽ��������NH3���������Ϊ20.0%����ʱH2��ת����Ϊ______������������һλС������
�⣺��1�����������漰�ķ�Ӧ�У�2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��ΪʹNO����������������ᣬӦͨ����������������漰�Ļ�ѧ��Ӧ�У���N2+3H2?2NH3��4NH3+5O2 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3�����Т٢ڢۢܢ�����������ԭ��Ӧ���ʴ�Ϊ��ʹNO������������ߡ����NO��ת���ʡ����� A B C��
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3�����Т٢ڢۢܢ�����������ԭ��Ӧ���ʴ�Ϊ��ʹNO������������ߡ����NO��ת���ʡ����� A B C��
��2����֪����4NH3��g��+3O2��g���T2N2��g��+6H2O��g������H=-1266.8kJ/mol
��N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol��
���ø�˹���ɢ�-2���ڿɵã�4NH3��g��+5O2��g�� 4NO��g��+6H2O��g������H=-905.8KJ/mol�����ݷ�Ӧ��
4NO��g��+6H2O��g������H=-905.8KJ/mol�����ݷ�Ӧ��
4NH3��g��+5O2��g�� 4NO��g��+6H2O��g������H=-905.8KJ/mol�� ת�Ƶĵ��ӵ����ʵ���
4NO��g��+6H2O��g������H=-905.8KJ/mol�� ת�Ƶĵ��ӵ����ʵ���
905.8KJ 20mol
Q 5mol
Q=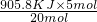 =226.45KJ��
=226.45KJ��
�ʴ�Ϊ��4NH3��g��+5O2��g�� 4NO��g��+6H2O��g������H=-905.8KJ/mol�� 226.45��
4NO��g��+6H2O��g������H=-905.8KJ/mol�� 226.45��
��3����Ӧ�ܷ��Է�����ȡ���ڷ�Ӧ�е��ʱ���ر��Լ���Ӧ�¶ȵĹ�ϵ������H-T?��S��0�����С�HΪ��Ӧ�ȣ�TΪ�¶ȣ���SΪ�ر䣩������ij��Ӧ�ġ�H��0���÷�Ӧ��һ�����Է����У��ʴ�Ϊ����һ����
��4��Ϊ���������ת���ʣ�Ӧ�Ƿ�Ӧ������Ӧ�����ƶ��������пɲ�ȡ�Ĵ�ʩ�У�����ѹǿ��ѭ�����úͲ��ϲ��䵪������ʱ������������ʴ�Ϊ��C D E��
��5���跴Ӧ��ʼʱH2��N2�����ʵ����ֱ�Ϊ3mol��1mol��ƽ��ʱת����xmolN2��
N2 +3H2 ?2NH3
��ʼ 1mol 3mol 0
ת�� x 3x 2x
ƽ�� 1-x 3-3x 2x
��������֪�� =20.0%����x=
=20.0%����x=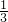 �����Դ�ʱH2��ת����Ϊ33.3%���ʴ�Ϊ��33.3%
�����Դ�ʱH2��ת����Ϊ33.3%���ʴ�Ϊ��33.3%
�������������漰�Ļ�ѧ��Ӧ�У���N2+3H2?2NH3��4NH3+5O2 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3
��1�����������漰�ķ�Ӧ�У�2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��ΪʹNO����������������ᣬӦͨ��������������ݷ�Ӧ�и����ʵ����Ԫ�صĻ��ϼ��Ƿ����仯���ж��Ƿ���������ԭ��Ӧ��
��2����֪����4NH3��g��+3O2��g���T2N2��g��+6H2O��g������H=-1266.8kJ/mol
��N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol�����ø�˹���ɿ���֪��Ӧ�ȣ����ݵ���ת�Ƶ����ʵ������㷴Ӧ�����ķ�Ӧ������ʵ��������μ��㷴Ӧ�ȣ�
��3����Ӧ�ܷ��Է�����ȡ���ڡ�H-T?��S��0�����С�HΪ��Ӧ�ȣ�TΪ�¶ȣ���SΪ�ر䣩��
��4��Ϊ���ijһ��Ӧ���ת���ʣ�Ӧʹƽ��������Ӧ�����ƶ���
��5�����ݰ����ӵ����ɽ�ϻ�ѧ����ʽ��������ʽ���㷨���㣮
���������⿼�鹤ҵ�ϳ�����李���˹���ɵ�Ӧ���Լ���ѧƽ�����⣬��Ŀ��Ϊ�ۺϣ�����ʱע���������⣬һ�Ǹ�˹���ɵ�Ӧ�ã���һ���ǻ�ѧƽ��ļ��㣮
 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3�����Т٢ڢۢܢ�����������ԭ��Ӧ���ʴ�Ϊ��ʹNO������������ߡ����NO��ת���ʡ����� A B C��
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3�����Т٢ڢۢܢ�����������ԭ��Ӧ���ʴ�Ϊ��ʹNO������������ߡ����NO��ת���ʡ����� A B C����2����֪����4NH3��g��+3O2��g���T2N2��g��+6H2O��g������H=-1266.8kJ/mol
��N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol��
���ø�˹���ɢ�-2���ڿɵã�4NH3��g��+5O2��g��
 4NO��g��+6H2O��g������H=-905.8KJ/mol�����ݷ�Ӧ��
4NO��g��+6H2O��g������H=-905.8KJ/mol�����ݷ�Ӧ��4NH3��g��+5O2��g��
 4NO��g��+6H2O��g������H=-905.8KJ/mol�� ת�Ƶĵ��ӵ����ʵ���
4NO��g��+6H2O��g������H=-905.8KJ/mol�� ת�Ƶĵ��ӵ����ʵ���905.8KJ 20mol
Q 5mol
Q=
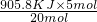 =226.45KJ��
=226.45KJ���ʴ�Ϊ��4NH3��g��+5O2��g��
 4NO��g��+6H2O��g������H=-905.8KJ/mol�� 226.45��
4NO��g��+6H2O��g������H=-905.8KJ/mol�� 226.45����3����Ӧ�ܷ��Է�����ȡ���ڷ�Ӧ�е��ʱ���ر��Լ���Ӧ�¶ȵĹ�ϵ������H-T?��S��0�����С�HΪ��Ӧ�ȣ�TΪ�¶ȣ���SΪ�ر䣩������ij��Ӧ�ġ�H��0���÷�Ӧ��һ�����Է����У��ʴ�Ϊ����һ����
��4��Ϊ���������ת���ʣ�Ӧ�Ƿ�Ӧ������Ӧ�����ƶ��������пɲ�ȡ�Ĵ�ʩ�У�����ѹǿ��ѭ�����úͲ��ϲ��䵪������ʱ������������ʴ�Ϊ��C D E��
��5���跴Ӧ��ʼʱH2��N2�����ʵ����ֱ�Ϊ3mol��1mol��ƽ��ʱת����xmolN2��
N2 +3H2 ?2NH3
��ʼ 1mol 3mol 0
ת�� x 3x 2x
ƽ�� 1-x 3-3x 2x
��������֪��
 =20.0%����x=
=20.0%����x=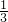 �����Դ�ʱH2��ת����Ϊ33.3%���ʴ�Ϊ��33.3%
�����Դ�ʱH2��ת����Ϊ33.3%���ʴ�Ϊ��33.3%�������������漰�Ļ�ѧ��Ӧ�У���N2+3H2?2NH3��4NH3+5O2
 4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3
4NO+6H2O��2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��NH3+HNO3�TNH4NO3��1�����������漰�ķ�Ӧ�У�2NO+O2�T2NO2��3NO2+H2O�T2HNO3+NO��4NO2+O2+H2O�T4HNO3��ΪʹNO����������������ᣬӦͨ��������������ݷ�Ӧ�и����ʵ����Ԫ�صĻ��ϼ��Ƿ����仯���ж��Ƿ���������ԭ��Ӧ��
��2����֪����4NH3��g��+3O2��g���T2N2��g��+6H2O��g������H=-1266.8kJ/mol
��N2��g��+O2��g���T2NO��g������H=+180.5kJ/mol�����ø�˹���ɿ���֪��Ӧ�ȣ����ݵ���ת�Ƶ����ʵ������㷴Ӧ�����ķ�Ӧ������ʵ��������μ��㷴Ӧ�ȣ�
��3����Ӧ�ܷ��Է�����ȡ���ڡ�H-T?��S��0�����С�HΪ��Ӧ�ȣ�TΪ�¶ȣ���SΪ�ر䣩��
��4��Ϊ���ijһ��Ӧ���ת���ʣ�Ӧʹƽ��������Ӧ�����ƶ���
��5�����ݰ����ӵ����ɽ�ϻ�ѧ����ʽ��������ʽ���㷨���㣮
���������⿼�鹤ҵ�ϳ�����李���˹���ɵ�Ӧ���Լ���ѧƽ�����⣬��Ŀ��Ϊ�ۺϣ�����ʱע���������⣬һ�Ǹ�˹���ɵ�Ӧ�ã���һ���ǻ�ѧƽ��ļ��㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ