��Ŀ����
��Һ��ijЩ����һ�������Ե����ӵ�Ũ�ȵIJⶨ�����ǣ�ȡһ���������Һ���������м��������ľ��ữ��KI��Һ��I-�����������±�������I2��Ȼ������֪Ũ�ȵ�Na2S2O3��Һ���еζ���������Ӧ��I2��2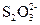 ====2I-��
====2I-��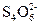 ���ⶨI2�������Ӷ�����������������ӵ�Ũ�ȡ�
���ⶨI2�������Ӷ�����������������ӵ�Ũ�ȡ�
��1�����ϵζ�������Ҫ����__________����ѡ����ѡ����Ϊָʾ�����жϵζ��յ㣬�ζ��յ��������__________��
��2����֪Cu2��������I-������Ӧ��2Cu2��4I-====2CuI��I2����ȡ20.00 mLijCuCl2��Һ���������������������0.11 mol��L-1��Na2S2O3��Һ20.00 mL����CuCl2��Һ�����ʵ���Ũ��Ϊ__________mol��L-1��
��3��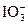 Ҳ������I-�������·�Ӧ��5I-��
Ҳ������I-�������·�Ӧ��5I-��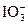 ��6H��====3I2��3H2O��ijѧ��ȡCu��IO3��2������Һ100 mL�����������ữ��KI��Һ����0.11 mol��L-1��Na2S2O3��Һ���еζ���������Na2S2O3��Һ35.30 mL���й�ϵʽCu��IO3��2��6.5I2����Cu��IO3��2��Һ�����ʵ���Ũ��Ϊ__________mol��L-1��
��6H��====3I2��3H2O��ijѧ��ȡCu��IO3��2������Һ100 mL�����������ữ��KI��Һ����0.11 mol��L-1��Na2S2O3��Һ���еζ���������Na2S2O3��Һ35.30 mL���й�ϵʽCu��IO3��2��6.5I2����Cu��IO3��2��Һ�����ʵ���Ũ��Ϊ__________mol��L-1��
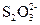 ====2I-��
====2I-��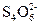 ���ⶨI2�������Ӷ�����������������ӵ�Ũ�ȡ�
���ⶨI2�������Ӷ�����������������ӵ�Ũ�ȡ���1�����ϵζ�������Ҫ����__________����ѡ����ѡ����Ϊָʾ�����жϵζ��յ㣬�ζ��յ��������__________��
| A����̪��Һ |
| B��KMnO4������Һ |
| C��������Һ |
| D��������Һ |
��3��
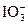 Ҳ������I-�������·�Ӧ��5I-��
Ҳ������I-�������·�Ӧ��5I-��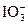 ��6H��====3I2��3H2O��ijѧ��ȡCu��IO3��2������Һ100 mL�����������ữ��KI��Һ����0.11 mol��L-1��Na2S2O3��Һ���еζ���������Na2S2O3��Һ35.30 mL���й�ϵʽCu��IO3��2��6.5I2����Cu��IO3��2��Һ�����ʵ���Ũ��Ϊ__________mol��L-1��
��6H��====3I2��3H2O��ijѧ��ȡCu��IO3��2������Һ100 mL�����������ữ��KI��Һ����0.11 mol��L-1��Na2S2O3��Һ���еζ���������Na2S2O3��Һ35.30 mL���й�ϵʽCu��IO3��2��6.5I2����Cu��IO3��2��Һ�����ʵ���Ũ��Ϊ__________mol��L-1����1��C����Һ����ɫǡ�ñ����ɫ���Ұ�����ڲ��ָ�
��2��0.11����3��0.003
��2��0.11����3��0.003
��1���õ�����Һ��ָʾ������ʼʱ��Һ����ɫ������Һ�еĵⵥ��������ʱ����Һ��Ϊ��ɫ������ж�ǡ����ȫ��Ӧ����������Һ����ɫǡ�ñ�Ϊ��ɫ���Ұ�����ڲ��ָ���
��2�����ݻ�ѧ����ʽ֪n��I2��=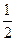 n��Na2S2O3��=
n��Na2S2O3��=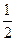 ��0.11 mol��L-1��20.0��10-3 L=1.1��10-3 mol���������ɵⵥ�ʵĻ�ѧ����ʽ֪2Cu2����I2������n��CuCl2��=2.2��10-3 mol��c��CuCl2��=
��0.11 mol��L-1��20.0��10-3 L=1.1��10-3 mol���������ɵⵥ�ʵĻ�ѧ����ʽ֪2Cu2����I2������n��CuCl2��=2.2��10-3 mol��c��CuCl2��=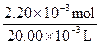 ="0.11" mol��L-1��
="0.11" mol��L-1��
��3�����ݻ�ѧ����ʽ֪n��I2��=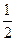 n��Na2S2O3��=
n��Na2S2O3��=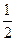 ��0.11 mol��L-1��35.30��10-3 L=1.94��10-3 mol���������ɵⵥ�ʵĹ�ϵʽCu��IO3��2��6.5I2��
��0.11 mol��L-1��35.30��10-3 L=1.94��10-3 mol���������ɵⵥ�ʵĹ�ϵʽCu��IO3��2��6.5I2��
����n��Cu��IO3��2��=2.99��10-4 mol��c��Cu��IO3��2��=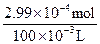 ="0.003" mol��L-1��
="0.003" mol��L-1��
��2�����ݻ�ѧ����ʽ֪n��I2��=
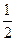 n��Na2S2O3��=
n��Na2S2O3��=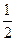 ��0.11 mol��L-1��20.0��10-3 L=1.1��10-3 mol���������ɵⵥ�ʵĻ�ѧ����ʽ֪2Cu2����I2������n��CuCl2��=2.2��10-3 mol��c��CuCl2��=
��0.11 mol��L-1��20.0��10-3 L=1.1��10-3 mol���������ɵⵥ�ʵĻ�ѧ����ʽ֪2Cu2����I2������n��CuCl2��=2.2��10-3 mol��c��CuCl2��=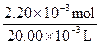 ="0.11" mol��L-1��
="0.11" mol��L-1����3�����ݻ�ѧ����ʽ֪n��I2��=
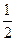 n��Na2S2O3��=
n��Na2S2O3��=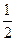 ��0.11 mol��L-1��35.30��10-3 L=1.94��10-3 mol���������ɵⵥ�ʵĹ�ϵʽCu��IO3��2��6.5I2��
��0.11 mol��L-1��35.30��10-3 L=1.94��10-3 mol���������ɵⵥ�ʵĹ�ϵʽCu��IO3��2��6.5I2������n��Cu��IO3��2��=2.99��10-4 mol��c��Cu��IO3��2��=
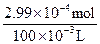 ="0.003" mol��L-1��
="0.003" mol��L-1��
��ϰ��ϵ�д�
�����Ŀ
 xC��g����ƽ��ʱ��������A��B��C�����ʵ���֮�Ⱦ�Ϊ
xC��g����ƽ��ʱ��������A��B��C�����ʵ���֮�Ⱦ�Ϊ