��Ŀ����
�����£�CH3COOH��Ka=1.7��10-5mol?L-1��NH3?H2O��Kb=1.7��10-5 mol?L-1������˵������ȷ���ǣ�������
������A����ͬ�¶��£���ͬŨ�ȵIJ�ͬ����ʣ�����볣����ͬ�������̶���ͬ��
B�������¶ȴٽ�������ʵ��룻
C�������£�������Һ��c��H+��=
����ˮ��Һ��c��OH-��=
��
D�������£�������Һ��c��H+��=
����ˮ��Һ��c��OH-��=
��
B�������¶ȴٽ�������ʵ��룻
C�������£�������Һ��c��H+��=
| Kac(CH3COOH) |
| Kbc(NH3?H2O) |
D�������£�������Һ��c��H+��=
| Kac(CH3COOH) |
| Kbc(NH3?H2O) |
����⣺A�������£�������Һ��c��H+��=
����ˮ��Һ��c��OH-��=
�����ߵ�Ũ�Ⱥ͵���ƽ�ⳣ����ȣ����Դ�����Һ��������Ũ�ȵ��ڰ�ˮ��Һ������������Ũ�ȣ�����ߵĵ���̶���ͬ����A��ȷ��
B�������¶ȴٽ������һˮ�ϰ����룬��Ka��Kb������B����
C�������£�������Һ��c��H+��=
����ˮ��Һ��c��OH-��=
��������Һ��Ũ����Դ�Сδ֪���������жϴ�����Һ��������Ũ�ȺͰ�ˮ������������Ũ�ȵ���Դ�С����C����
D�������£�������Һ��c��H+��=
����ˮ��Һ��c��OH-��=
��������Һ��Ũ�Ⱥ͵���ƽ�ⳣ����ͬ�����Դ�����Һ��������Ũ�ȵ��ڰ�ˮ��Һ������������Ũ�ȣ���D��ȷ��
��ѡ��BC��
| Kac(CH3COOH) |
| Kbc(NH3?H2O) |
B�������¶ȴٽ������һˮ�ϰ����룬��Ka��Kb������B����
C�������£�������Һ��c��H+��=
| Kac(CH3COOH) |
| Kbc(NH3?H2O) |
D�������£�������Һ��c��H+��=
| Kac(CH3COOH) |
| Kbc(NH3?H2O) |
��ѡ��BC��
���������⿼����������ʵĵ��룬��ȷ������ʵĵ����ص㼰����������Ũ�Ⱥͼ�������������Ũ�ȵļ��㷽�����ɽ��ע�����ƽ�ⳣ��ֻ���¶��йأ�����Һ��Ũ���أ�Ϊ�״��㣮

��ϰ��ϵ�д�
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д� ABC����ȫ�ž�ϵ�д�
ABC����ȫ�ž�ϵ�д�
�����Ŀ
��������10mL pH=3�Ĵ�����Һ�м���ˮϡ�ͺ����и�����ֵ��С���ǣ�������
| A������ĵ���̶� | ||
| B����Һ�ĵ����� | ||
| C����Һ��c��OH-�� | ||
D����Һ��
|
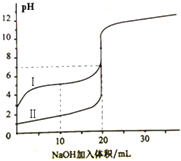 ��2013?������һģ�������£���0.100mol/L NaOH ��Һ�ֱ�ζ�20.00mL 0.100mol/L������ʹ��ᣬ�ζ�
��2013?������һģ�������£���0.100mol/L NaOH ��Һ�ֱ�ζ�20.00mL 0.100mol/L������ʹ��ᣬ�ζ�