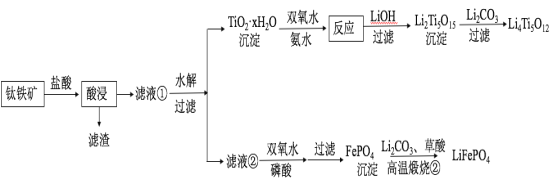
��Ŀ����
����Ŀ��I.��ͼΪ�����������װ�ã��Իش�
![]()

��1���������ҳ�����Ƭ�϶�������B��_________����缫���ϣ����缫��Ӧʽ��_________��Ӧѡ�õĵ������Һ��_____________��
��2���������ҳؽ��д�ͭ�ĵ�⾫������________�����A����B�����Ǵ�ͭ������ͭ�л�����Au��Ag��Fe�������ڵ����еĴ�����ʽ��λ��Ϊ_____________________��
��3�����ص���������̪��Һ�����һ��ʱ��___________���C����Fe�����������ʺ�ɫ��
��4��д���׳ظ����ĵ缫��Ӧʽ��________________________________�����׳�����3.2gCH3OH���壬������������Ϸų����������ʵ���Ϊ______________��
II.��5�������÷�ӦFe +2Fe3+= 3Fe2+���ԭ��ء�
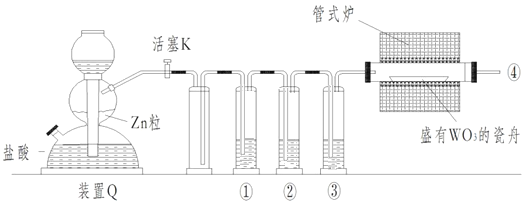
���Ҫ�ٸ�װ�þ��������ѧ��ת��Ϊ���ܵ�Ч�ʣ�
�ڲ��ϼ��������Һ��ѡ����ͼ������Ҫ��ע��
�ۻ������ӵ�ת�Ʒ���____________________________________
���𰸡� ��Ƭ Ag++ e-= Ag AgNO3��Һ A Au��Ag�Ե��ʵ���ʽ������c(����)�·���Fe��Fe2+����ʽ������Һ�� Fe CH3OH -6e-+ 8OH-= CO32-+ 6H2O 0.175mol 
���������������������ͼʾ��֪��A�Ǽ״�ȼ�ϵ�أ�ͨ��״��ĵ缫�Ǹ�����ͨ�������ĵ缫���������ҡ���Ϊ���أ��ҳ���A��������B������������������������ʯī��������
��������1�����ʱ�Ƽ����������Ʋ���������������жƲ����������Һ���������Һ���������ҳ�����Ƭ�϶�������B����Ƭ���缫��Ӧʽ��Ag++ e-= Ag��Ӧѡ�õĵ������Һ��AgNO3��Һ��
��2����ͭ����ʱ����ͭ����������ͭ������������ͭ���������Һ���������ҳؽ��д�ͭ�ĵ�⾫������A�Ǵ�ͭ������ͭ�л�����Au��Ag��Fe��Au��Ag������С��ͭ��Au��Ag����ʧ���ӣ�Au��Ag�Ե��ʵ���ʽ������c(����)�·���Fe�����Դ���ͭ��Fe����ʧ��������Fe2+������Fe��Fe2+����ʽ������Һ����
��3���������Զ��Ե缫���ʳ��ˮ��������Ӧʽ![]() ��������Ӧʽ
��������Ӧʽ![]() ������������������������ǿ������������̪��Һ�� Fe�����������������ʺ�ɫ��
������������������������ǿ������������̪��Һ�� Fe�����������������ʺ�ɫ��
��4���׳���ȼ�ϵ�أ�����ͨ��״��������缫��Ӧʽ��CH3OH -6e-+ 8OH-= CO32-+ 6H2O�����ݵ缫��Ӧ�����׳�����3.2gCH3OH������ת�Ƶ���0.6mol���������������η���![]() ��
��![]() ��Ӧ�����ݵ����غ��������غ���������������0.05mol������������0.125mol,���������Ϸų����������ʵ���Ϊ0.175mol��
��Ӧ�����ݵ����غ��������غ���������������0.05mol������������0.125mol,���������Ϸų����������ʵ���Ϊ0.175mol��
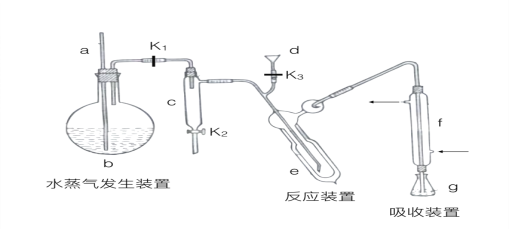
II.��5������Ҫ��װ����ͼ ��
��