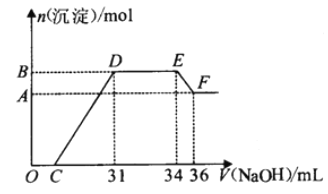
��Ŀ����
����Ŀ���ҹ��й����ĺ����ߣ���ˮ�ۺ����ô��п�Ϊ����ˮ���庬��Ϊ 65 mg/L���Ӻ� ˮ����ȡ��Ĺ�ҵ�������£�

��1�����ϲ�������ѻ������̬���壬������ֽ�֮ת��ɻ���̬���壬��Ŀ������_____��
��2�������ͨ���ȿ�����ˮ�������� Br2�����������________��
A��������B����ԭ��C���ӷ��� D����ʴ��
��3���������̢����漰�����ӷ�Ӧ���£��������淽���������ʵ��Ļ�ѧ��������_____
![]()
��4�����������д�������������Ҳ�����ö�������ˮ��Һ���գ�������������������д�� �����������ˮ��Һ��Ӧ�����ӷ���ʽ��________���ɴ˷�Ӧ��֪�� �����������⣬�ڹ�ҵ������Ӧ�������Ҫ������_______��
��5��ʵ���ҷ����廹�������ܼ���ȡ�������п������������ȡ������________��
A���Ҵ� B�����Ȼ�̼ C������������Һ D����
���𰸡� ������Ԫ�� C 3 3 1 5 3 SO2+Br2+2H2O=4H++2Br-+SO42- ǿ����豸�����ظ�ʴ BD
���������������̷�����֪����ˮͨ��һ�����������õ���ˮ��±ˮ��±ˮ��������������������Ϊ�����壬ͨ���ȿ�����ˮ��������Br2�����õ����嵥�ʵ��ӷ��ԣ�����������Һ����������Ӻ������ӷ���������ԭ��Ӧ�õ��嵥�ʣ�
��1�����������ѻ������̬����Ũ�Ⱥܵͣ����ֱ�����������ɱ��ϸߣ������ڹ�ҵ���������������ѻ������̬���壬�������ֽ�֮ת��ɻ���̬���壬��Ŀ���Ǹ�����Ԫ�أ����ͳɱ�����2�����ӷ���������ͨ���ȿ�����ˮ��������Br2������������Ļӷ��ԣ���ѡC����3���÷�Ӧ��BrԪ�ػ��ϼ���0�۱�Ϊ-1�ۡ�+5�ۣ�����С��������5���ٽ��ԭ���غ�����غ�÷���ʽΪ3Br2+3CO32-��BrO3-+5Br-+3CO2������4�����������д�������������Ҳ�����ö�������ˮ��Һ���գ������������������������������ˮ��Һ��Ӧ����������廯�⣬��Ӧ�Ļ�ѧ����ʽΪ��Br2+SO2+2H2O=2HBr+H2SO4�����ӷ���ʽSO2+Br2+2H2O��4H++2Br-+SO42-�������������Ϊǿ�ᣬǿ����豸�����ظ�ʴ�� ��5����ȡ����ѡȡ������ȡ�������ʲ���Ӧ����������ȡ���е��ܽ�ȴ�����ԭ�ܼ��е��ܽ�ȡ���ȡ����ԭ�ܼ������ܣ�A���Ҵ�������ˮ�����Բ�������ȡ����A����B�����Ȼ�̼������ȡ��ѡȡ��������������ȡ����B��ȷ��C������������Һ�����ܷ����ӳɷ�Ӧ�����Բ�������ȡ����C����D����������ȡ��ѡȡ��������������ȡ����D��ȷ����ѡBD��