��Ŀ����
�л���A��B����Է���������С��200����ȫȼ��ʱֻ����CO2��H2O��Bȼ��ʱ���ĵ����������ɵĶ�����̼�����ʵ�����ȡ�B��̼����Ԫ���ܵ������ķ���Ϊ46.67����B������������Ӧ������NaHCO3��Һ��Ӧ�ų�CO2��1molAˮ������1mol�������1molB��A��Һ�������ԣ�����FeCl3��Һ����ɫ��(1)A��B��Է�������֮��Ϊ________��
(2)B������Ӧ��________________����ԭ�ӡ�
(3)A�Ľṹ��ʽΪ_____�������� ___��____�������� ���� ____
(4)д��B��������������ͬ���칹��Ľṹ��ʽ��
__________________________________��__________________________________��
__________________________________��__________________________________��
�𰸣�
������
��ʾ��
������
| (1)104
(2)3 (3) (4)
|
��ʾ��
| B����Cn(H2O)m������֪��O�������ķ������Ӷ�֪��Ħ����������ȷ������ʽ��B��NaHCO3��Һ��Ӧ�ų�CO2����֪�д��ǻ��������ǻ���AΪ�������B���ɵ�����A���ǻ���
|

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ��
�� 
 ��
�� 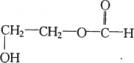 ��
��  ��
��